 Author
Author  Correspondence author
Correspondence author
Animal Molecular Breeding, 2024, Vol. 14, No. 3
Received: 27 Mar., 2024 Accepted: 27 May, 2024 Published: 30 May, 2024
Embryo transfer technology has been developing since the early 20th century and has gradually been applied in various animal breeding programs. In felines, the development and application of this technology began relatively late and faces many challenges, including the unique reproductive physiology and hormonal cycles of cats. With the rise of the pet economy and the increasing demand for purebred cats, optimizing these technologies not only meets market demands but also aids in scientific research and the conservation of endangered species. This study analyzes various embryo transfer techniques and influencing factors, aiming to explore and resolve key technological and ethical issues in feline embryo transfer. Through technical optimization and policy guidance, this research strives to advance the scientific development and market application of feline breeding programs
1 Introduction
Feline breeding programs play a crucial role in the conservation and management of both domestic and wild cat populations. These programs aim to maintain genetic diversity, support population sustainability, and mitigate the risks associated with inbreeding and habitat loss. The domestic cat (Felis catus) often serves as a model for developing and refining reproductive technologies that can be applied to endangered wild felids (Pope, 2000; Thongphakdee et al., 2020). The success of these programs relies heavily on a comprehensive understanding of feline reproductive biology and the implementation of advanced reproductive technologies.
Reproductive technologies, including artificial insemination (AI), in vitro fertilization (IVF), and embryo transfer (ET), have become indispensable tools in feline breeding programs. These technologies not only enhance natural breeding efforts but also provide alternative methods to overcome challenges such as breeding incompatibility and suboptimal environmental conditions (Farstad, 2000). The development and application of these techniques have shown promising results in various wild cat species, contributing to the birth of viable offspring and the preservation of genetic material (Gómez et al., 2009). Despite the progress, there remain significant challenges in optimizing these technologies to achieve consistent and efficient outcomes (Mains and Voorhis, 2010).
This study optimizes embryo transfer (ET) techniques in feline breeding programs by examining various technical aspects of the ET procedure, including the use of soft catheters, ultrasound guidance, and cervical mucus removal to improve pregnancy outcomes, addressing limitations and variability in ovarian stimulation, gamete quality, and embryo development, with the goal of enhancing ET efficiency and success rates in domestic and wild felines, ultimately contributing to the sustainability and genetic diversity of feline populations.
2 Background of Embryo Transfer in Felines
2.1 Historical development of embryo transfer techniques
Embryo transfer (ET) in felines has evolved significantly over the past few decades, driven by the need to preserve endangered species and improve breeding programs. Initially, the focus was on developing reliable methods for oocyte recovery, in vitro fertilization (IVF), and embryo cryopreservation. The domestic cat (Felis catus) has played a pivotal role as a model for these techniques, which have subsequently been applied to various wild cat species (Thongphakdee et al., 2010). Early successes included the birth of domestic kittens from embryos produced in vitro and transferred to recipient queens, demonstrating the feasibility of these techniques. More recent advancements have focused on optimizing cryoprotectant exposure techniques to improve the survival and developmental competence of vitrified oocytes and embryos (Tharasanit et al., 2011).
2.2 Comparison with other reproductive technologies
Embryo transfer is one of several assisted reproductive technologies (ART) used in feline breeding programs. Compared to other methods such as artificial insemination (AI) and somatic cell nuclear transfer (SCNT), ET offers unique advantages. For instance, ET allows for the transfer of embryos produced from genetically valuable or endangered individuals, thereby enhancing genetic diversity and conservation efforts (Zambelli and Cunto, 2022). In contrast, AI primarily focuses on the direct insemination of sperm into the female reproductive tract, which may not be as effective in species with complex reproductive physiology. SCNT, while promising, has shown limited success in felines, with challenges in achieving full-term development of cloned embryos (Swanson, 2012). ET, therefore, remains a critical tool in both domestic and wild feline breeding programs, offering a higher success rate and broader applicability.
2.3 Challenges specific to feline reproductive physiology
Feline reproductive physiology presents several challenges that complicate the application of embryo transfer techniques. One significant issue is the induction of ovulation, which in felines is typically triggered by mating. Hormonal treatments using eCG and hCG have been developed to induce ovulation in donor and recipient queens, but the timing and dosage must be carefully managed to ensure successful embryo recovery and implantation. Additionally, the maintenance of pregnancy in recipient queens, especially during the non-breeding season, requires the administration of sustained action progesterone to prevent spontaneous abortion (Tsutsui, 2000). Another challenge is the high sensitivity of feline oocytes and embryos to cryoinjury during vitrification, necessitating the development of optimized cryoprotectant exposure techniques to improve survival rates. These physiological complexities underscore the need for continued research and refinement of ET protocols to enhance their efficacy and reliability in feline breeding programs (Pelican et al., 2006).
By addressing these challenges and leveraging advancements in reproductive technologies, researchers aim to optimize embryo transfer techniques, thereby improving the outcomes of feline breeding programs and contributing to the conservation of endangered species.
3 Embryo Collection Techniques
3.1 Hormonal stimulation protocols
Hormonal stimulation is a critical step in optimizing oocyte retrieval in feline breeding programs. The use of follicle-stimulating hormone (FSH) has been shown to significantly enhance follicular development and oocyte yield (Table 1). For instance, studies on Eld's deer have demonstrated that FSH stimulation increases the main follicular diameter, although it does not necessarily increase the number of oocytes retrieved or their quality (Thongphakdee et al., 2017). In domestic cats, repeated FSH treatments have been effective in stimulating follicular development, with a mean recovery of 24.1 mature oocytes per laparoscopic retrieval. Additionally, minimal ovarian stimulation protocols combined with elective single embryo transfer have been shown to yield acceptable live birth rates in human IVF programs, suggesting potential applicability in feline breeding (Chinarov et al., 2021).
|
Table 1 MicroRNAs characterized in oocytes of rFSH and rFSH-rLH treatment protocols (Adopted from Konstantinidou et al., 2023) |
3.2 Oocyte retrieval methods
Oocyte retrieval methods vary, but laparoscopic ovum pick-up (LOPU) and transvaginal ovum pick-up (TVOPU) are commonly used. LOPU has been extensively used in cats, with over 1603 laparoscopic oocyte retrievals performed, resulting in the recovery of more than 38,000 mature oocytes (Pope, 2014). In cows, different OPU protocols have been tested, showing that dominant follicle removal followed by FSH treatment and subsequent OPU can significantly increase the number of follicles aspirated and oocytes retrieved (Konstantinidou et al., 2023). These methods can be adapted for use in feline species to optimize oocyte yield and quality.
3.3 Embryo culture and developmental stages
The culture and development of embryos are crucial for successful embryo transfer. Advances in cell culture media have led to improved outcomes in embryo development. For example, the use of optimized in vitro maturation (IVM) protocols has resulted in high maturation and fertilization rates, with significant blastocyst development (Junk and Yeap, 2012). In lions, IVM and intracytoplasmic sperm injection (ICSI) have been successfully performed, resulting in the development of blastocysts, although the developmental speed was slower compared to domestic cats (Fernandez-Gonzalez et al., 2015). Additionally, studies have shown that blastocyst stage transfers result in higher live birth rates compared to cleavage stage transfers, suggesting that extending embryo culture to the blastocyst stage could improve outcomes in feline breeding programs (Glujovsky et al., 2016).
By integrating these techniques, feline breeding programs can enhance the efficiency and success rates of embryo transfer, ultimately contributing to the conservation and propagation of endangered feline species.
4 Embryo Transfer Protocols
4.1 Selection of recipient queens
The selection of recipient queens is a critical step in optimizing embryo transfer (ET) techniques in feline breeding programs. Recipient queens should be in good health, have a proven reproductive history, and be free from any reproductive disorders. The body condition score (BCS) is an essential factor, as it influences the success of the ET program. Proper nutrition and health management are crucial to ensure that the recipient queens are in optimal condition to receive and support the transferred embryos (Sala et al., 2020).
4.2 Timing and synchronization of donor and recipient
Synchronization of the estrous cycles of donor and recipient queens is vital for the success of ET. Fixed-time embryo transfer (FTET) protocols, which eliminate the need for estrus detection, have been shown to improve synchronization and increase the efficiency of ET programs. These protocols often involve the use of gonadotrophin-releasing hormone (GnRH), prostaglandin F2α (PGF2α), and progesterone-releasing devices to control follicular wave emergence and ovulation (Baruselli et al., 2010; Baruselli et al., 2011). The timing of hormone administration is crucial to ensure that the recipient queen's uterine environment is synchronized with the developmental stage of the transferred embryos (Sala et al., 2020).
4.3 Embryo transfer procedures
The ET procedure involves the careful handling and transfer of embryos into the recipient queen's uterus. Non-surgical methods are preferred due to their lower risk and higher acceptance among breeders. The use of ultrasonography to guide the transfer process can improve the accuracy and success rates of ET. It is essential to ensure that the recipient queen has a functional corpus luteum (CL) at the time of transfer, as this is critical for maintaining pregnancy (Bonacker et al., 2020). The quality and stage of the embryos also play a significant role in the success of the transfer, with blastocyst stage embryos often showing better outcomes compared to earlier stages (Baruselli et al., 2010).
4.4 Post-transfer care and monitoring
Post-transfer care and monitoring are essential to ensure the success of the ET program. Recipient queens should be monitored for signs of estrus and pregnancy, with regular ultrasonographic examinations to confirm the presence and development of embryos. Proper nutrition and stress management are crucial during this period to support the queen's health and the development of the embryos. Additionally, monitoring progesterone levels can help in assessing the queen's readiness to support pregnancy and in making necessary adjustments to the care regimen (Phillips et al., 2016; Wiltbank et al., 2019).
By following these optimized protocols, feline breeding programs can improve the efficiency and success rates of embryo transfer, ultimately contributing to better genetic management and breeding outcomes.
5 Factors Influencing Embryo Transfer Success
5.1 Genetic and health status of donor and recipient
The genetic and health status of both the donor and recipient cats play a crucial role in the success of embryo transfer. Studies have shown that the reproductive status and health of the donor significantly affect the quality and viability of the embryos produced (Figure 1). For instance, non-lactating donor cows produced a larger number of viable embryos compared to lactating cows, indicating that the physiological state of the donor can impact embryo quality (Lamas et al., 2020). Additionally, recipient health, including uterine pathology and endometrial thickness, has been identified as a critical determinant of successful embryo implantation and pregnancy outcomes.
 Figure 1 The effect of sire and semen concentration (Adopted from Currin et al., 2022) |
The two charts from Currin et al. (2022) clearly demonstrate the impact of the donor bull and semen concentration on embryo development in in vitro fertilization (IVF). Chart A illustrates how the genetic background of different bulls significantly affects embryo cleavage, the embryo/oocyte ratio, and the embryo/cleaved embryo ratio. This reflects the importance of the donor's genetic health in the success rate of IVF. Chart B further explores the effect of semen concentration on a specific donor bull (Bull D), showing variability in how semen concentration influences embryo development, which may be related to the donor's reproductive health and genetic quality. These findings emphasize the importance of selecting donors with healthy and superior genetic traits to optimize IVF outcomes.
5.2 Quality of embryos
The quality of the embryos being transferred is another pivotal factor. High-grade embryos have been associated with higher pregnancy rates. For example, in bovine studies, the quality of embryos (graded on a scale) was directly correlated with pregnancy per embryo transfer (P/ET) rates, with higher quality embryos resulting in better outcomes (Ferraz et al., 2016). Similarly, in equine and ovine studies, the age and developmental stage of the embryo at the time of transfer were significant predictors of successful pregnancy establishment (Currin et al., 2022).
5.3 Environmental and management conditions
Environmental and management conditions, including season, temperature, and stress levels, significantly influence embryo transfer success. Heat stress, for instance, has been shown to reduce the number of viable embryos and lower pregnancy rates in cattle (Chebel et al., 2008; Tandulwadkar et al., 2019). Proper management practices, such as the use of heat abatement systems and minimizing stress through adequate facilities, are essential to optimize the conditions for both donors and recipients.
5.4 Role of nutrition and veterinary care
Nutrition and veterinary care are fundamental to the success of embryo transfer programs. Adequate nutrition ensures that both donor and recipient animals are in optimal condition for reproduction. For example, prepartum nutrition in cattle is critical to ensure that cows calve in adequate body condition, which is necessary for the reinitiation of postpartum estrous cycles and successful synchronization protocols (Phillips and Jahnke, 2016). Veterinary care, including regular health checks and management of reproductive health issues, is also crucial. Conditions such as metritis and calving problems have been shown to negatively impact pregnancy rates in cattle.
6 Advances in Embryo Transfer Technology
6.1 Cryopreservation of embryos
Cryopreservation has revolutionized the field of reproductive biotechnology by enabling the long-term storage of embryos, which is crucial for genetic preservation and breeding programs. Recent advancements in cryopreservation techniques, such as vitrification, have shown promising results in various species, including felines. Vitrification, a rapid freezing method, has been particularly effective in preserving the viability of immature cat oocytes, although it still presents challenges in terms of meiotic and developmental competence compared to non-vitrified controls (Tharasanit et al., 2011). Additionally, the use of stepwise cryoprotectant exposure techniques has improved the outcomes of vitrified immature cat oocytes, leading to successful pregnancies following embryo transfer (Michailov et al., 2023). These advancements highlight the potential of cryopreservation in enhancing feline breeding programs by allowing for the storage and transport of genetic material across different locations and time periods.
6.2 In vitro fertilization (IVF) and its integration
In vitro fertilization (IVF) has become a cornerstone of assisted reproductive technologies, offering a controlled environment for fertilization and early embryo development. The integration of IVF with embryo transfer techniques has shown significant improvements in pregnancy rates and genetic selection. For instance, the use of IVF in conjunction with cryopreservation has been demonstrated to enhance genetic selection and optimize cross-breeding schemes in cattle, which can be translated to feline breeding programs (Mogas, 2018). Moreover, advancements in cell culture media have shifted IVF practices towards blastocyst stage transfers, which have been associated with higher live birth rates compared to early cleavage stage transfers (Glujovsky et al., 2022). This shift allows for better synchronization between the embryo and the uterine environment, potentially increasing the success rates of embryo transfers in felines.
6.3 Use of genetic screening and selection
Genetic screening and selection have become integral components of modern breeding programs, enabling the identification and transfer of the most viable embryos. Techniques such as preimplantation genetic diagnosis (PGD) and genomic testing are being developed to screen embryos for genetic abnormalities and desirable traits before transfer. In the context of feline breeding, these techniques can help in selecting embryos with the highest implantation potential, thereby improving the overall success rates of breeding programs (Owen et al., 2022). Additionally, the ability to cryopreserve and subsequently transfer all embryos without impairing pregnancy rates suggests that genetic screening can be effectively integrated with cryopreservation to optimize breeding outcomes (Hasler, 2014).
6.4 Future trends in feline reproductive biotechnology
The future of feline reproductive biotechnology is poised to benefit from several emerging trends and technologies. One such trend is the refinement of invasive fertilization techniques, such as intracytoplasmic sperm injection (ICSI), which can facilitate the transfer of selected embryonic or somatic cell donor nuclei into oocytes, offering new avenues for genetic modification and preservation. Another promising area is the development of reliable systems for in vitro production of embryos, which, when coupled with advanced cryopreservation methods, can significantly enhance the efficiency and success rates of feline breeding programs. Furthermore, the integration of genomic testing with embryo transfer techniques is likely to become more prevalent, allowing for more precise selection of embryos and potentially reducing the number of transfers required to achieve successful pregnancies (Owen et al., 2022). These advancements underscore the potential for continued innovation and improvement in feline reproductive biotechnology, ultimately contributing to the conservation and enhancement of feline genetic diversity.
7 Ethical Considerations in Feline Embryo Transfer
7.1 Welfare of donor and recipient cats
The welfare of both donor and recipient cats is paramount in the practice of embryo transfer. Ensuring minimal stress and discomfort during procedures is essential. Techniques such as unilateral transfers using a low number of embryos have been shown to improve the welfare of recipient females by reducing the invasiveness of the procedure (Lamas et al., 2020). Additionally, the use of less invasive techniques can help in reducing the number of donor and recipient animals required, thereby aligning with the principles of the 3Rs (Replacement, Reduction, and Refinement) (Lamas et al., 2020). The stepwise cryoprotectant exposure technique has also been developed to minimize cryoinjury to oocytes, which is crucial for maintaining the health and viability of embryos.
7.2 Implications for genetic diversity
Embryo transfer techniques have significant implications for genetic diversity within feline populations. The propagation of select female genomes through embryo transfer can lead to rapid genetic gains, similar to the effects seen with artificial insemination in other species (Phillips and Jahnke, 2016). However, this practice must be carefully managed to avoid reducing genetic diversity, which is particularly critical in conservation efforts for endangered felids. The use of domestic cats as recipients for embryos from wild cat species can aid in the conservation of genetic material from endangered species, but it also necessitates careful consideration of genetic compatibility and diversity.
7.3 Regulatory and breeding guidelines
Adherence to regulatory and breeding guidelines is essential to ensure ethical practices in feline embryo transfer. Regulations often focus on the welfare of the animals involved, the ethical implications of genetic manipulation, and the necessity of maintaining genetic diversity. Ethical considerations in gamete and embryo donation, such as consent, screening, and assessment of donors and recipients, are also critical. These guidelines help ensure that the practices not only advance scientific and breeding goals but also respect the ethical standards of animal welfare and genetic integrity.
8 Case Study
8.1 Case study: successful implementation of embryo transfer in a feline breeding program
In a recent feline breeding program, the implementation of embryo transfer (ET) techniques was explored to enhance genetic diversity and improve reproductive outcomes. The program utilized vitrified immature cat oocytes (Figure 2), which were subjected to a stepwise cryoprotectant exposure technique. This method aimed to protect the oocytes against cryoinjury during vitrification, thereby preserving their viability for subsequent in vitro maturation and fertilization (Colombo et al., 2020a). The embryos derived from these oocytes were then transferred into recipient queens, resulting in successful pregnancies. This case study highlights the potential of advanced ET techniques in feline breeding programs, demonstrating their efficacy in achieving successful pregnancies and contributing to the genetic management of valuable or endangered feline species.
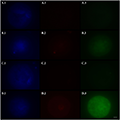 Figure 2 Representative fluorescence micrographs of vitrified (A,B), fresh (C, negative control), and hydrogen peroxide-treated (D, positive control) domestic cat oocytes (Adopted from Colombo et al. 2020b) Image caption: Bright blue fluorescence (A.1,B.1,C.1,D.1) indicates the nuclear material. Bright red fluorescence (B.2,D.2) in the nuclear area indicates DNA fragmentation by TUNEL assay. Green fluorescence in the ooplasm (A.3,B.3,C.3,D.3) indicates, according to its intensity (Adopted from Colombo et al. 2020b) |
8.2 Challenges encountered and solutions implemented
During the implementation of the ET program, several challenges were encountered. One significant issue was the reduction in meiotic and developmental competence of vitrified immature cat oocytes compared to non-vitrified controls. To address this, the program experimented with different cryoprotectant agents and exposure techniques. It was found that using ethylene glycol (EG) alone or in combination with dimethyl sulfoxide (DMSO) yielded higher maturation rates than DMSO alone, regardless of the equilibration technique used (Sala et al., 2020). Additionally, the program faced difficulties in ensuring the proper deposition of embryos within the uterine cavity. This was mitigated by employing ultrasound guidance and using soft catheters to minimize uterine contractility and avoid negative pressure from the catheter (Nowak et al., 2020). These adjustments were crucial in overcoming the technical challenges and optimizing the ET procedure.
8.3 Outcomes and lessons learned
The outcomes of the ET program were promising, with successful pregnancies established in recipient queens receiving embryos derived from both non-vitrified and vitrified/warmed immature oocytes (Ma et al., 2021). This success underscores the importance of optimizing cryoprotectant exposure techniques and ensuring precise embryo deposition within the uterus. The program also highlighted the need for continuous monitoring and adjustment of ET protocols to address specific challenges encountered during the process. Key lessons learned include the critical role of cryoprotectant selection and exposure methods in preserving oocyte viability, as well as the importance of gentle and precise embryo transfer techniques to enhance pregnancy outcomes. These insights can inform future ET programs in feline breeding, contributing to the advancement of reproductive technologies and the conservation of genetic diversity in feline populations.
9 Concluding Remarks
The research on optimizing embryo transfer (ET) techniques in feline breeding programs has highlighted several critical factors that can significantly influence the success rates of ET procedures. Key findings include the importance of using soft catheters and gentle manipulation to avoid uterine contractility, the benefits of ultrasound guidance, and the necessity of removing cervical mucus to prevent embryo loss during catheter withdrawal. Additionally, the deposition of embryos in the midportion of the uterus and avoiding negative pressure from the catheter have been shown to improve outcomes. The use of multiple ovulation and embryo transfer (MOET) techniques has also been identified as a powerful tool for genetic improvement, allowing for more intense and accurate selection, and shorter generation intervals.
The findings from this research have significant implications for feline breeding programs. By adopting optimized ET techniques, breeders can enhance the success rates of embryo transfers, leading to more efficient and effective breeding programs. The use of soft catheters, ultrasound guidance, and proper handling techniques can minimize the risk of uterine contractility and embryo loss, thereby increasing the likelihood of successful pregnancies. Furthermore, the application of MOET can accelerate genetic improvement in feline populations, similar to its impact on sheep and cattle breeding programs. This can result in healthier and genetically superior feline breeds, benefiting both breeders and pet owners.
Future research should focus on further refining ET techniques to maximize success rates and minimize risks. Studies should explore the optimal conditions for embryo transfer in different feline breeds, including the ideal number of embryos to transfer and the best practices for handling and transferring embryos. Additionally, research should investigate the long-term genetic impacts of using MOET in feline breeding programs, including potential risks such as increased inbreeding. Practical recommendations for breeders include adopting the use of soft catheters, performing trial transfers, and utilizing ultrasound guidance to ensure accurate embryo placement. By implementing these practices, breeders can improve the efficiency and success of their breeding programs, ultimately contributing to the advancement of feline genetics and health.
Acknowledgements
Author would like to express our gratitude to the two anonymous peer reviewers for their critical assessment and constructive suggestions on our manuscript.
Conflict of Interest Disclosure
Author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Baruselli P., Ferreira R., Filho M., Nasser L., Rodrigues C., and Bó G., 2010, Bovine embryo transfer recipient synchronisation and management in tropical environments, Reproduction Fertility and Development, 22(1): 67-74.
https://doi.org/10.1071/RD09214
Baruselli P., Ferreira R., Sales J., Gimenes L., Filho M., Martins C., Rodrigues C., and Bó G., 2011, Timed embryo transfer programs for management of donor and recipient cattle, Theriogenology, 76(9): 1583-1593.
https://doi.org/10.1016/j.theriogenology.2011.06.006
Bonacker R., Gray K., Breiner C., Anderson J., Patterson D., Spinka C., and Thomas J., 2020, Comparison of the 7 and 7 Synch protocol and the 7-day CO-Synch + CIDR protocol among recipient beef cows in an embryo transfer program, Theriogenology, 158: 490-496.
https://doi.org/10.1016/J.THERIOGENOLOGY.2020.09.033
Chebel R., Demétrio D., and Metzger J., 2008, Factors affecting success of embryo collection and transfer in large dairy herds, Theriogenology, 69(1): 98-106.
https://doi.org/10.1016/J.THERIOGENOLOGY.2007.09.008
Chinarov R.Y., Singina G.N., Havlicek V., Taradajnic N.P., Taradajnic T.E., and Pozyabin S.V., 2021, 123 Efficiency of embryo production using ovum pickup oocytes recovered from stimulated and nonstimulated cows, Reproduction Fertility and Development, 33: 169-170.
https://doi.org/10.1071/RDV33N2AB123
Colombo M., Zahmel J., Binder C., Herbel J., Luvoni G., and Jewgenow K., 2020a, Ovary cold storage and shipment affect oocyte yield and cleavage rate of cat immature vitrified oocytes, Cryobiology, 98: 181-186.
https://doi.org/10.1016/j.cryobiol.2020.11.003
PMID: 33171197
Colombo M., Zahmel J., Jänsch S., Jewgenow K., and Luvoni G.C., 2020b, Inhibition of apoptotic pathways improves DNA integrity but not developmental competence of domestic cat immature vitrified oocytes, Frontiers in Veterinary Science, 7: 588334.
https://doi.org/10.3389/fvets.2020.588334
Currin L., Baldassarre H., de Macedo M.P., Glanzner W.G., Gutierrez K., Lazaris K., Guay V., Herrera M.E.C., da Silva Z., Brown C., Joron E., Herron R., and Bordignon V., 2022, Factors affecting the efficiency of in vitro embryo production in prepubertal mediterranean water buffalo, Animals, 12(24): 3549.
https://doi.org/10.3390/ani12243549
PMID: 36552466 PMCID: PMC9774791
Farstad W., 2000, Current state in biotechnology in canine and feline reproduction, Animal Reproduction Science, 60-61: 375-387.
https://doi.org/10.1016/S0378-4320(00)00106-8
PMID: 10844209
Fernandez-Gonzalez L., Hribal R., Stagegaard J., Zahmel J, and Jewgenow K., 2015, Production of lion (Panthera leo) blastocysts after in vitro maturation of oocytes and intracytoplasmic sperm injection, Theriogenology, 83(6): 995-999.
https://doi.org/10.1016/j.theriogenology.2014.11.037
PMID: 25586639
Ferraz P.A., Burnley C., Karanja J., Viera-Neto A., Santos J.E.P., Chebel R., and Galvão K., 2016, Factors affecting the success of a large embryo transfer program in Holstein cattle in a commercial herd in the southeast region of the United States, Theriogenology, 86(7): 1834-1841.
https://doi.org/10.1016/j.theriogenology.2016.05.032
Glujovsky D., Farquhar C., Retamar A.M.Q., Sedo C.R.A., and Blake D., 2016, Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology, The Cochrane Database of Systematic Reviews, 6: CD002118.
https://doi.org/10.1002/14651858.CD002118.pub5
Glujovsky D., Retamar A., Sedo C., Ciapponi A., Cornelisse S., and Blake D., 2022, Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology, The Cochrane Database of Systematic Reviews, 5(5): CD002118.
https://doi.org/10.1002/14651858.CD002118.pub6
PMID: 35588094 PMCID: PMC9119424
Gómez M.C., Pope C.E., Ricks D.M., Lyons J., Dumas C., and Dresser B.L., 2009, Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer, Reproduction fertility and development, 21(1): 76-82.
https://doi.org/10.1071/RD08222
PMID: 19152748
Hasler J.F., 2014, Forty years of embryo transfer in cattle: a review focusing on the journal Theriogenology the growth of the industry in North America and personal reminisces, Theriogenology, 81(1): 152-169.
https://doi.org/10.1016/j.theriogenology.2013.09.010
Junk S.M., and Yeap D., 2012, Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome, Fertility and Sterility, 98(4): 888-892.
https://doi.org/10.1016/j.fertnstert.2012.06.055
PMID: 22835445
Konstantinidou F., Placidi M., Emidio G., Stuppia L., Tatone C., Gatta V., and Artini P.G., 2023, Maternal microRNA profile changes when LH is added to the ovarian stimulation protocol: a pilot study, Epigenomes, 7(4): 25.
https://doi.org/10.3390/epigenomes7040025
PMID: 37873810 PMCID: PMC10594432
Lamas S., Franquinho F., Morgado M., Mesquita J.R., Gärtner F., and Amorim I., 2020, C57BL/6J and B6129F1 embryo transfer: unilateral and bilateral transfer embryo number and recipient female background control for the optimization of embryo survival and litter size, Animals, 10(8): 1424.
https://doi.org/10.3390/ani10081424
PMID: 32824021 PMCID: PMC7459990
Ma L.L., Li Z., Ma Z.R., Ma J.B., and Zhao F., 2021, Immunization against inhibin promotes fertility in cattle: a meta-analysis and quality assessment, Frontiers in Veterinary Science, 8: 687923.
https://doi.org/10.3389/fvets.2021.687923
PMID: 34621805 PMCID: PMC8490720
Mains L., and van Voorhis B.J., 2010, Optimizing the technique of embryo transfer, Fertility and Sterility, 94(3): 785-790.
https://doi.org/10.1016/j.fertnstert.2010.03.030
PMID: 20409543
Michailov Y., Friedler S., and Saar-Ryss B., 2023, Methods to improve frozen-thawed blastocyst transfer outcomes- the IVF laboratory perspective, Journal of IVF-Worldwide, 1(3-1): 1-5.
https://doi.org/10.46989/001c.87541
Mogas T., 2018, Update on the vitrification of bovine oocytes and invitro-produced embryos, Reproduction Fertility and Development, 31(1): 105-117.
https://doi.org/10.1071/RD18345
Nowak A., Kochan J., Świętek E., Kij B., Prochowska S., Witarski W., Bugno-Poniewierska M., and Niżański W., 2020, Survivability and developmental competences of domestic cat Felis catus) oocytes after Cryotech method vitrification, Reproduction in domestic animals, Zuchthygiene, 55(8): 992-997.
https://doi.org/10.1111/rda.13741
PMID: 32516454
Owen C.M., Roberts M.A., Long K.A., Gumber D., Barceló-Fimbres M., Altermatt J.L., and Campos-Chillon L.F., 2022, Novel synthetic oviductal fluid for conventional freezing 1 (SCF1) culture medium improves development and cryotolerance of in vitro produced holstein embryos, Journal of Animal Science, 100(3): skac043.
https://doi.org/10.1093/jas/skac043
PMID: 35148394 PMCID: PMC8919821
Pelican K., Wildt D., Pukazhenthi B., and Howard J., 2006, Ovarian control for assisted reproduction in the domestic cat and wild felids, Theriogenology, 66(1): 37-48.
https://doi.org/10.1016/J.THERIOGENOLOGY.2006.03.013
PMID: 16630653
Phillips P., and Jahnke M., 2016, Embryo Transfer Techniques Donors and Recipients), The Veterinary clinics of North America., Food animal Practice, 32(2): 365-385.
https://doi.org/10.1016/j.cvfa.2016.01.008
Pope C., 2000, Embryo technology in conservation efforts for endangered felids, Theriogenology, 53(1): 163-174.
https://doi.org/10.1016/S0093-691X(99)00249-6
PMID: 10735071
Pope C., 2014, Aspects of in vivo oocyte production blastocyst development and embryo transfer in the cat, Theriogenology, 81(1): 126-137.
https://doi.org/10.1016/j.theriogenology.2013.09.006
Sala R., Carrenho-Sala L., Absalón-Medina V., Lopez A., Fosado M., Moreno J., Wiltbank M., and Garcia-Guerra A., 2020, 105 Optimization of a five-day fixed-time embryo transfer program in dairy heifers: Use of gonadotrophin-releasing hormone at initiation of the protocol, Reproduction Fertility and Development, 32(2): 179.
https://doi.org/10.1071/rdv32n2ab105
Sala R., Melo L., Motta J., Leffers-Neto L., Carrenho-Sala L., Fosado M., Moreno J., Baruselli P., Wiltbank M., and Garcia-Guerra A., 2020, Optimization of a 5-day fixed-time embryo transfer FTET) protocol in heifers I., Manipulation of circulating progesterone through reutilization of intravaginal progesterone devices during FTET, Theriogenology, 156: 171-180.
https://doi.org/10.1016/j.theriogenology.2020.06.002
Swanson W., 2012, Laparoscopic oviductal embryo transfer and artificial insemination in felids--challenges strategies and successes, reproduction in domestic animals, Zuchthygiene, 47(Suppl 6): 136-140.
https://doi.org/10.1111/rda.12069
Tandulwadkar S., Patil M., and Naik S., 2019, Optimising the outcome of embryo transfer, EMJ Reproductive Health, 5(1); 110-119.
https://doi.org/10.33590/emjreprohealth/10310436
Tharasanit T., Manee-in S., Buarpung S., Chatdarong K., Lohachit C., and Techakumphu M., 2011, Successful pregnancy following transfer of feline embryos derived from vitrified immature cat oocytes using 'stepwise' cryoprotectant exposure technique, Theriogenology, 76(8): 1442-1449.
https://doi.org/10.1016/j.theriogenology.2011.06.014
PMID: 21820721
Thongphakdee A., Berg D., Tharasanit T., Thongtip N., Tipkantha W., Punkong C., Tongthainan D., Noimoon S., Maikeaw U., Kajornklin N., Siriaroonrat B., Comizzoli P., and Kamolnorranath S., 2017, The impact of ovarian stimulation protocol on oocyte quality subsequent in vitro embryo development and pregnancy after transfer to recipients in Eld's deer (Rucervus eldii thamin), Theriogenology, 91: 134-144.
https://doi.org/10.1016/j.theriogenology.2016.12.021
Thongphakdee A., Siriaroonrat B., Manee-in S., Klincumhom N., Kamolnorranath S., Chatdarong K., and Techakumphu M., 2010, Intergeneric somatic cell nucleus transfer in marbled cat and flat-headed cat, Theriogenology, 73(1): 120-128.
https://doi.org/10.1016/j.theriogenology.2009.09.001
PMID: 19880167
Thongphakdee A., Sukparangsi W., Comizzoli P., and Chatdarong K., 2020, Reproductive biology and biotechnologies in wild felids, Theriogenology, 150: 360-373.
https://doi.org/10.1016/j.theriogenology.2020.02.004
PMID: 32102745
Tsutsui T., Yamane I., Hattori I., Kurosawa N., Matsunaga H., Murao I., Kanda M., and Hori T., 2000, Feline embryo transfer during the non-breeding season, The Journal of Veterinary Medical Science, 62(11): 1169-1175.
https://doi.org/10.1292/JVMS.62.1169
PMID: 11129860
Wiltbank M., Garcia-Guerra A., Sala R.V., Fosada M., Carrenho-Sala L., and Moreno J., 2019, 200 Improving efficiency of embryo transfer ET) programs by optimizing fertility and management of recipients, Journal of Animal Science, 97(Suppl 2): 116-117.
https://doi.org/10.1093/JAS/SKZ122.206
Zambelli D., and Cunto M., 2022, Artificial insemination in queens in the clinical practice setting: protocols and challenges, Journal of Feline Medicine and Surgery, 24: 871-880.
https://doi.org/10.1177/1098612X221118756
PMID: 36002144 PMCID: PMC10812221

. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Xiaofang Lin
Related articles
. Embryo transfer
. Feline
. Reproductive technology
. Genetic diversity
. Animal welfare
Tools
. Post a comment
.png)


