 Author
Author  Correspondence author
Correspondence author
Animal Molecular Breeding, 2024, Vol. 14, No. 5
Received: 15 Aug., 2024 Accepted: 21 Sep., 2024 Published: 14 Oct., 2024
Breeding practices in domestic cats have significantly shaped their genetic diversity, impacting the prevalence of hereditary diseases within specific breeds. This study examines the role of selective breeding, inbreeding, and breed popularity in contributing to the incidence of genetic disorders in cats, also analyzed the genetic health of various cat breeds, focusing on patterns of inheritance, common mutations, and the impact of genetic screening programs. Additionally, a case study on Breed X provides insights into the relationship between breeding practices and disease prevalence, exploring management strategies and lessons that can inform future breeding programs. The findings underscore the physiological, behavioral, and care-related consequences of hereditary diseases on cat health, with broader implications for breeders, owners, and veterinary practitioners. Reducing the prevalence of these diseases presents challenges, including the need for breeder awareness, improved access to genetic testing, and addressing regulatory and ethical considerations. Looking forward, advancements in genetic screening and breeding technologies offer promising avenues for enhancing the genetic health of cats through sustainable breeding practices. Sustainable, ethically informed breeding practices are crucial for promoting the long-term genetic health and well-being of cat populations.
1 Introduction
The breeding of domestic cats has long been a subject of interest for both pet enthusiasts and scientific researchers. While selective breeding has led to the development of distinct cat breeds with desirable traits, it has also inadvertently increased the prevalence of hereditary diseases (Holst, 2022). Cat breeding practices have evolved significantly over the years, with a focus on maintaining breed standards and enhancing specific physical and behavioral traits. However, the small population size within specific breeds often necessitates a degree of inbreeding to preserve these traits, which can lead to a higher incidence of hereditary diseases (Plitman et al., 2019; Casal, 2022). For instance, the prevalence of autosomal dominant polycystic kidney disease (ADPKD) in Persian cats is a direct consequence of such breeding practices (Michel-Regalado et al., 2022). Additionally, the extent of linkage disequilibrium (LD) in various cat breeds reflects their unique breeding histories and strategies, further influencing the genetic health of these populations (Alhaddad et al., 2013).
Understanding hereditary diseases in cat populations is crucial for several reasons. Firstly, it allows for the identification and management of genetic disorders that can significantly impact the health and welfare of cats. For example, a study on the epidemiology of presumed hereditary ocular diseases in cats in France highlighted the prevalence of conditions such as entropion, corneal sequestration, and retinal dysplasia, which are more common in certain breeds (Bott and Chahory, 2022). Secondly, knowledge of genetic mutations and their effects can inform breeding decisions, helping to reduce the incidence of these diseases. The largest DNA-based study of domestic cats to date has shown that DNA panel testing can effectively identify disease-associated variants, aiding in the selection of healthier breeding stocks (Anderson et al., 2021). Lastly, understanding the genetic diversity within and between cat breeds can help mitigate the negative effects of inbreeding, thereby improving overall feline health (Gandolfi and Alhaddad, 2015).
This study investigates the impact of breeding practices on the prevalence of genetic diseases in cats by examining the prevalence of specific genetic disorders across various cat breeds, analyzing their genetic diversity, evaluating the effectiveness of current breeding strategies in mitigating these diseases, and exploring the roles of genetic testing and genome-wide association studies in identifying and managing these diseases, aiming to promote the development of more effective breeding programs that prioritize feline health and welfare through a comprehensive overview of feline genetics and breeding practices.
2 Breeding Practices and Genetic Health
2.1 Selective breeding and its impacts
Selective breeding in cats aims to enhance desirable traits, but it can also inadvertently propagate genetic disorders. Gene mapping projects have revealed that selective breeding practices sometimes lead to structural alterations that may be harmful. For instance, inherited bone and cartilage disorders have been linked to specific genetic mutations, which are often perpetuated through selective breeding (Haase et al., 2016). This practice, while beneficial for maintaining breed standards, necessitates a careful balance to avoid the propagation of hereditary diseases.
2.2 Inbreeding and the prevalence of genetic disorders
Inbreeding is a common practice in cat breeding due to the small population sizes within breeds. This practice is essential to maintain breed standards but can lead to significant genetic health issues. Inbreeding depression, characterized by decreased fertility, smaller litter sizes, and increased neonatal mortality, is a notable consequence (Casal, 2022). Studies on traditional Siamese cats have shown that inbreeding can spread hereditary diseases and reduce genetic diversity. Optimal contribution selection and minimizing kinship within regional subpopulations can help improve genetic diversity, although achieving this without a supervised breeding program is challenging. Comprehensive records and DNA analysis can aid breeders in making informed decisions to mitigate the negative impacts of inbreeding.
2.3 Popular breeds and associated hereditary diseases
Certain cat breeds are more prone to specific hereditary diseases due to their genetic makeup (Junaid, 2011; Vapalahti et al., 2016). For example, the traditional Siamese cat, which has been subject to extensive inbreeding, shows a higher prevalence of hereditary diseases (Pistorius and Blokker, 2021). Additionally, selective breeding has been linked to inherited skeletal conditions in various breeds, highlighting the need for veterinarians to understand these genetic bases to improve diagnosis and management. Effective breeding practices, including the use of DNA panels and genetic counseling, are crucial in reducing the incidence of these hereditary diseases and maintaining the overall health of popular cat breeds (Rokhsar et al., 2021).
3 Mechanisms of Hereditary Disease Transmission in Cats
3.1 Genetic inheritance patterns in cats
Genetic inheritance in cats follows the basic principles of Mendelian genetics, where traits are passed from parents to offspring through genes. These genes can be dominant, recessive, or linked to sex chromosomes. For instance, the study on genome sequencing in feline colonies highlights how genetic diversity and inbreeding coefficients can be managed to avoid the spread of hereditary diseases (Farias et al., 2017). Additionally, the extent of linkage disequilibrium (LD) in domestic cats, which varies significantly among breeds, plays a crucial role in understanding how genetic traits are inherited and can be used to design efficient genome-wide association studies (Alhaddad et al., 2013).
3.2 Common genetic mutations leading to diseases
Cats are known to carry over 70 genetic mutations that can lead to various diseases and structural abnormalities. These mutations include those affecting bone and cartilage, as well as other clinically relevant health concerns (Haase et al., 2016). For example, the study on mucopolysaccharidosis VI (MPS VI) in cats discusses specific DNA variants such as L476P and D520N in the ARSB gene, which are associated with this condition (Lyons et al., 2016). Furthermore, a large-scale study involving over 11 000 domestic cats identified 13 disease-associated variants across 48 breeds, underscoring the prevalence and impact of these mutations on feline health (Anderson et al., 2021).
3.3 Role of breed-specific genetic testing
Breed-specific genetic testing is essential for managing and reducing the incidence of hereditary diseases in cats. The use of whole genome sequencing (WGS) and other genetic tests allows for the identification of disease-causing variants, enabling breeders to make informed decisions to maintain genetic diversity and avoid inbreeding (Lyons, 2015). For instance, the study on the genetic epidemiology of blood type and disease variants in cats demonstrates how panel testing can help reduce the frequency of disease-associated variants in pedigreed cat populations. Additionally, the investigation into inherited diseases in cats emphasizes the importance of genetic testing as a routine diagnostic tool in feline healthcare, which can significantly improve the management and prevention of hereditary conditions (Gandolfi and Alhaddad, 2015).
4 Consequences of Hereditary Diseases on Cat Health
4.1 Physiological and behavioral impacts
Hereditary diseases in cats can have profound physiological and behavioral impacts. For instance, inbreeding, which is often necessary to maintain breed standards, can lead to decreased fertility, smaller litter sizes, increased neonatal illness, and higher neonatal mortality rates (Casal, 2022). Additionally, specific hereditary ocular diseases such as entropion, corneal sequestration, persistent pupillary membrane, cataracts, and retinal dysplasia have been identified in certain breeds, leading to significant visual impairments and discomfort (Bott and Chahory, 2022). These conditions not only affect the physical health of the cats but can also lead to behavioral changes due to pain or discomfort, such as increased irritability or withdrawal.
4.2 Veterinary and long-term care needs
Cats with hereditary diseases often require extensive veterinary care and long-term management. For example, the management of reproductive health in catteries necessitates close collaboration between veterinarians and breeders to address issues such as sanitation, hygiene, and infectious disease control, which are critical for maintaining the health of breeding cats (Goericke-Pesch and Packeiser, 2022). Moreover, cats with hereditary ocular diseases may need regular veterinary check-ups, surgical interventions, and ongoing treatments to manage their conditions (Wang and Lin, 2024). The need for specialized care and monitoring can place a significant burden on veterinary resources and require breeders to maintain detailed health records and breeding outcomes.
4.3 Implications for cat owners and breeders
The presence of hereditary diseases in cats has significant implications for both cat owners and breeders. Owners of cats with hereditary conditions may face increased veterinary costs and the emotional burden of managing a pet with chronic health issues. For breeders, the challenge lies in making informed breeding choices to minimize the incidence of hereditary diseases (Matsumoto et al., 2020). Utilizing DNA panels to estimate the coefficient of inbreeding and maintaining comprehensive breeding records are essential practices to mitigate these risks. Additionally, the longevity and mortality data indicate that crossbred cats tend to live longer than purebred cats, suggesting that breeding practices that promote genetic diversity could enhance the overall health and lifespan of cats (O'Neill et al., 2015). This information underscores the importance of responsible breeding practices and the need for ongoing education and collaboration between breeders and veterinarians to improve the health outcomes of feline populations.
5 Case Study
5.1 Background of selected cat breeds and health outcomes
The study of hereditary diseases in cats has revealed significant insights into the prevalence and impact of genetic disorders across various breeds. For instance, a retrospective study in France identified that 11.1% of cats presented with presumed hereditary or breed-related ocular diseases, with conditions such as entropion, corneal sequestration, persistent pupillary membrane, cataract, and retinal dysplasia being the most common (Figure 1) (Bott and Chahory, 2022). Additionally, the Burmese breed has been noted for its unusually high incidence of type 2 diabetes (T2D), particularly in Australian-bred Burmese (ABB) cats, which exhibit a four-fold increase in T2D incidence compared to their American counterparts (Samaha et al., 2019; Balmer et al., 2020). These findings underscore the importance of understanding breed-specific health outcomes to inform breeding practices and improve feline health management.
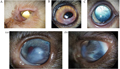 Figure 1 Cat eye related diseases (Adapted from Bott and Chahory, 2022) Image caption: A: Primary inferotemporal entropion with trichiasis and mucopurulent discharge in a 1-year-old male Maine Coon cat (affected bilaterally); B: Corneal sequestration in an 8-year-old female Persian cat; C: Persistent pupillary membrane in a 2-month-old male domestic shorthair cat; Anterior segment dysgenesis in a 5-month-old male domestic shorthair cat: (a) front view; and (b) lateral view (Adapted from Bott and Chahory, 2022) |
5.2 Analysis of breeding practices in Breed X
Breed X, identified here as the Burmese breed, has been extensively studied for its genetic predisposition to T2D. The high incidence of T2D in ABB cats is likely due to a genetic founder effect, which has been confirmed through genome-wide association studies identifying specific single-nucleotide polymorphisms (SNPs) associated with the disease (Table 1). Furthermore, the extent of linkage disequilibrium (LD) in Burmese cats is notably long, indicating a high degree of genetic similarity within the breed, which can exacerbate the prevalence of hereditary diseases (Alhaddad et al., 2013). This genetic homogeneity is a direct consequence of selective breeding practices aimed at maintaining breed standards, which inadvertently increase the risk of hereditary diseases.
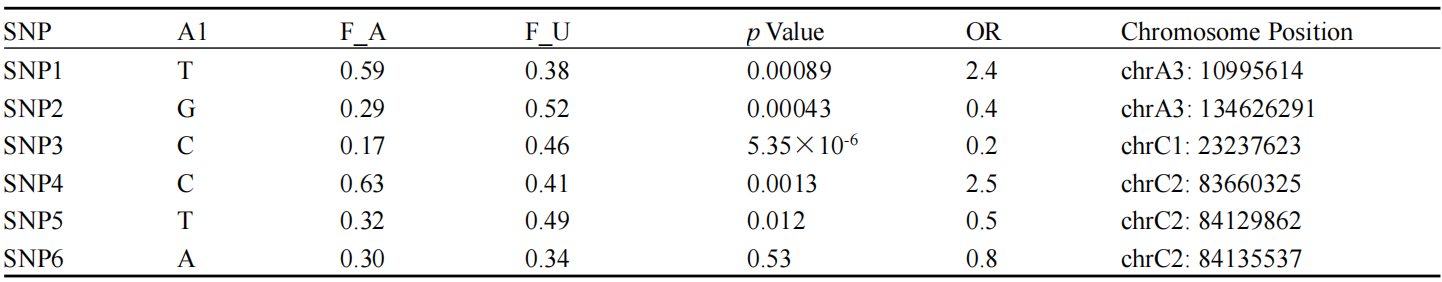 Table 1 Testing candidate SNPs in a validation cohort. DNA samples from a further 37 diabetic ABB cats, 47 normoglycaemic Australian-bred Burmese cats, and 84 Burmese cats from the low-prevalence US population were genotyped at the SNPs implicated by the GWAS. Results were analysed using Plink. A1 indicates the minor allele (i.e., least frequently observed based on the whole sample); F_A and F_U indicate the frequency of the minor allele in the affected and unaffected cats, respectively. The p value is shown calculated using Fisher’s Exact test and Bonferroni correction. No odds ratio could be calculated for SNP2 because the associated allele was not found in the non-diabetic cats (Adopted from Balmer et al., 2020) |
5.3 Disease prevalence and management strategies
The prevalence of hereditary diseases in cats varies significantly across breeds. For example, the prevalence of entropion in Persians and Maine Coons is 2.2%, while corneal sequestration is more common in Persians and Exotic Shorthairs, with a prevalence of 2.4%. In the case of Burmese cats, the high incidence of T2D necessitates targeted management strategies. Genetic screening and selective breeding practices are crucial in managing these hereditary conditions. The use of genome sequencing data has proven effective in identifying disease-causing variants and managing genetic diversity within feline colonies (Farias et al., 2017). Additionally, comprehensive breeding records and DNA panels can help breeders make informed decisions to reduce the incidence of hereditary diseases.
5.4 Lessons learned and recommendations
The case study of Breed X highlights several critical lessons and recommendations for managing hereditary diseases in cats. First, the importance of genetic screening cannot be overstated. Regular screening for known disease-causing variants can help identify at-risk individuals and inform breeding decisions to minimize the spread of these conditions. Second, maintaining genetic diversity within breeds is essential. Breeding practices should aim to avoid excessive inbreeding, which can lead to decreased fertility and increased neonatal mortality (Figure 2) (Casal, 2022). Finally, collaboration between breeders, veterinarians, and geneticists is vital. By working together, these stakeholders can develop and implement effective strategies to manage hereditary diseases and improve the overall health and well-being of cat populations.
 Figure 2 Box and whisker plots showing the correlation between the size of kitten litters and coefficient of inbreeding (F) for 74 litters sired by a single tom over a 7-year and 9-month period. There is a decrease in the median litter size (indicated by arrows) as the F value increases. N=number of litters per group; x=mean litter size; whiskers=highest and lowest numbers of kittens per litter; box=75% of all litter sizes (Adopted from Casal, 2022) |
6 Challenges in Reducing Hereditary Diseases in Cats
6.1 Breeder knowledge and awareness
One of the primary challenges in reducing hereditary diseases in cats is the level of knowledge and awareness among breeders. Many breeders may not be fully informed about the genetic implications of their breeding choices. For instance, inbreeding, which is often practiced to maintain breed standards, can lead to decreased fertility and increased neonatal mortality (Casal, 2022). Additionally, while some breeders are well-informed and actively share knowledge via the internet and social media, there is still a significant gap in understanding the complexities of feline genetics and the impact of inbreeding on health (Goericke-Pesch and Packeiser, 2022). Comprehensive records on breeding outcomes and the use of DNA panels to estimate the coefficient of inbreeding are recommended to make informed breeding choices.
6.2 Access to genetic testing and resources
Access to genetic testing and resources is another significant challenge. Although commercially available DNA panels can inform breeding choices and help estimate genetic diversity, not all breeders have access to these tools or the financial means to utilize them. The availability of genetic testing has increased, with over 70 genetic mutations identified in cats, many of which are clinically relevant (Gandolfi and Alhaddad, 2015; Lyons, 2015). However, the implementation of these tests in routine breeding practices is still limited. The largest DNA-based study of domestic cats highlighted the importance of panel testing across populations to reduce disease-associated genetic variants (Anderson et al., 2021). Despite these advancements, the cost and accessibility of genetic testing remain barriers for many breeders.
6.3 Regulatory and ethical considerations
Regulatory and ethical considerations also pose challenges in reducing hereditary diseases in cats. There is a need for more systematic screening and regulation to ensure that breeding practices do not perpetuate genetic diseases. For example, the widespread distribution of the rdAc allele, which causes retinal degeneration, underscores the need for regulatory measures to control its prevalence in certain breeds (Menotti-Raymond et al., 2010; Ofri et al., 2015). Ethical considerations also come into play when deciding which genetic traits to prioritize and which to eliminate. The debate over genetic testing for mucopolysaccharidosis Type VI (MPS VI) in cats illustrates the ethical dilemma of reducing gene pools versus preventing disease (Lyons et al., 2016). Breeders and veterinarians must navigate these complex ethical landscapes to make decisions that balance the health and genetic diversity of cat populations.
7 Future Directions in Ethical Cat Breeding
7.1 Advances in genetic screening and breeding programs
Advancements in genetic screening and breeding programs are pivotal in reducing the incidence of hereditary diseases in cats. The integration of whole-genome sequencing (WGS) into routine diagnostic tools is becoming increasingly feasible and is expected to revolutionize feline healthcare by enabling the identification of genetic mutations associated with various diseases (Lyons, 2015). For instance, the use of WGS in a feline colony has demonstrated its potential in identifying disease-causing variants and managing genetic diversity effectively, thereby preventing the spread of hereditary diseases (Farias et al., 2017). Additionally, commercially available DNA panels can estimate the coefficient of inbreeding, aiding breeders in making informed decisions to maintain genetic health and reduce inbreeding-related fertility issues. The implementation of these advanced genetic tools can significantly enhance the health outcomes of breeding programs by allowing for the strategic selection of breeding pairs to avoid the propagation of deleterious genes.
7.2 Role of technology in monitoring health outcomes
The role of technology in monitoring health outcomes is increasingly critical in ethical cat breeding. The use of digital health records and genetic databases allows for the comprehensive tracking of breeding outcomes, including the health and vitality of kittens from birth to weaning (Casal, 2022). This data-driven approach enables breeders and veterinarians to identify patterns and potential health issues early, facilitating timely interventions. Moreover, the application of genome sequencing data in managing feline pedigrees has shown promise in maintaining genetic diversity and monitoring the presence of harmful genetic variants. (Si, 2024) By leveraging these technological advancements, breeders can ensure more accurate and efficient monitoring of health outcomes, ultimately leading to healthier cat populations (Goericke-Pesch and Packeiser, 2022).
7.3 Policy recommendations and advocacy
To promote ethical breeding practices, it is essential to establish robust policy recommendations and advocacy efforts. Policies should mandate the use of genetic testing and screening for known hereditary diseases before breeding, ensuring that only healthy cats are used in breeding programs (Gandolfi and Alhaddad, 2015). Additionally, guidelines should be developed to prevent the unnecessary reduction of gene pools, as seen in the case of the MPS VI D520N variant, where unwarranted genetic testing could lead to inbreeding depression (Lyons et al., 2016). Advocacy efforts should focus on educating breeders and the public about the importance of genetic diversity and the risks associated with inbreeding. Collaboration between veterinarians and breeders is crucial in this regard, as it fosters a shared understanding and commitment to improving feline health and wellbeing. By implementing these policy recommendations and advocating for responsible breeding practices, the incidence of hereditary diseases in cats can be significantly reduced, ensuring healthier and more resilient cat populations (Bott and Chahory, 2015).
8 Concluding Remarks
The research on the impact of breeding practices on the incidence of hereditary diseases in cats has revealed several critical insights. Selective breeding practices have been shown to contribute to the prevalence of various genetic disorders. For instance, the study on bone and cartilage disorders in cats highlights that selective breeding can lead to harmful structural alterations and inherited skeletal conditions. Additionally, a significant proportion of ocular diseases in cats, such as entropion and corneal sequestration, have been linked to hereditary factors, with certain breeds like Persians and Maine Coons being over-represented. The Burmese cat has been identified as a genetic model for type 2 diabetes, demonstrating how selective breeding can lead to a higher incidence of specific diseases within certain breeds. Furthermore, the analysis of genetic diversity and inbreeding practices in cat populations indicates that close inbreeding is prevalent and can lead to decreased fertility and increased neonatal mortality. The use of genome sequencing data has been shown to be beneficial in managing breeding programs to avoid the spread of inherited disorders and maintain genetic diversity.
The findings underscore the importance of sustainable breeding practices to mitigate the incidence of hereditary diseases in cats. Sustainable breeding involves maintaining genetic diversity and avoiding close inbreeding, which has been linked to various health issues such as decreased fertility and increased susceptibility to diseases. The use of genetic tools and genome sequencing can aid in identifying disease-causing variants and managing pedigrees to ensure healthy breeding outcomes. For example, the study on the traditional Siamese cat population suggests that optimal contribution selection and limiting contributions from other breeds can improve genetic diversity and reduce the spread of hereditary diseases. Additionally, systematic screening and genetic testing can help in early detection and prevention of hereditary conditions, as recommended for ocular diseases in cats.
Promoting genetic health in cats requires a multifaceted approach that includes responsible breeding practices, genetic testing, and ongoing research. Breeders should be encouraged to keep comprehensive records and utilize available genetic tools to make informed breeding decisions. Veterinarians play a crucial role in advising breeders and monitoring the health of cat populations, ensuring that genetic diversity is maintained and hereditary diseases are minimized. Collaborative efforts between breeders, veterinarians, and researchers are essential to develop and implement strategies that promote the long-term genetic health of cat populations. By prioritizing sustainable breeding practices and leveraging advancements in genetic research, we can work towards reducing the incidence of hereditary diseases and improving the overall health and well-being of cats.
Acknowledgements
I would like to thank two anonymous review experts for their support.
Conflict of Interest Disclosure
Author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Alhaddad H., Khan R., Grahn R., Gandolfi B., Mullikin J., Cole S., Gruffydd-Jones T., Häggström J., Lohi H., Longeri M., and Lyons L., 2013, Extent of linkage disequilibrium in the domestic cat, felis silvestris catus, and its breeds, PLoS ONE, 8(1): e53537.
https://doi.org/10.1371/journal.pone.0053537
PMid:23308248 PMCid:PMC3538540
Anderson H., Davison S., Lytle K., Honkanen L., Freyer J., Mathlin J., Kyöstilä K., Inman L., Louviere A., Foran R., Forman O., Lohi H., and Donner J., 2021, Genetic epidemiology of blood type, disease and trait variants, and genome-wide genetic diversity in over 11 000 domestic cats, PLoS Genetics, 18(6): e1009804.
https://doi.org/10.1371/journal.pgen.1009804
PMid:35709088 PMCid:PMC9202916
Balmer L., O'Leary C., Menotti-Raymond M., David V., O’Brien S., Penglis B., Hendrickson S., Reeves-Johnson M., Gottlieb S., Fleeman L., Vankan D., Rand J., and Morahan G., 2020, Mapping of diabetes susceptibility loci in a domestic cat breed with an unusually high incidence of diabetes mellitus, Genes, 11(11): 1369.
https://doi.org/10.3390/genes11111369
PMid:33228033 PMCid:PMC7699364
Bott M., and Chahory S., 2022, Epidemiology and clinical presentation of feline presumed hereditary or breed-related ocular diseases in France: retrospective study of 129 cats, Journal of Feline Medicine and Surgery, 24: 1274-1282.
https://doi.org/10.1177/1098612X221080598
PMid:35257624 PMCid:PMC10812340
Casal M., 2022, Feline fertility: consequences of inbreeding and implications for reproductive fitness, Journal of Feline Medicine and Surgery, 24: 847-852.
https://doi.org/10.1177/1098612X221118755
PMid:36002141 PMCid:PMC10812227
Farias F., Tomlinson C., Labuda J., Pérez-Camargo G., Middleton R., and Warren W., 2017, The practical use of genome sequencing data in the management of a feline colony pedigree, BMC Veterinary Research, 13: 225.
https://doi.org/10.1186/s12917-017-1144-y
PMid:28750619 PMCid:PMC5532773
Gandolfi B., and Alhaddad H., 2015, Investigation of inherited diseases in cats, Journal of Feline Medicine and Surgery, 17: 405-415.
https://doi.org/10.1177/1098612X15581133
PMid:25896240 PMCid:PMC10816245
Goericke-Pesch S., and Packeiser E., 2022, Reproductive Management in Catteries: Optimising health and wellbeing through veterinarian-breeder collaboration, Journal of Feline Medicine and Surgery, 24: 881-904.
https://doi.org/10.1177/1098612X221118760
PMid:36002135 PMCid:PMC10812226
Haase B., Mazrier H., and Wade C., 2016, Digging for known genetic mutations underlying inherited bone and cartilage characteristics and disorders in the dog and cat, Veterinary and Comparative Orthopaedics and Traumatology, 29: 269-276.
https://doi.org/10.3415/VCOT-16-02-0037
PMid:27189647
Holst B., 2022, Feline breeding and pregnancy management: what is normal and when to intervene, Journal of Feline Medicine and Surgery, 24: 221-231.
https://doi.org/10.1177/1098612X221079708
PMid:35209770 PMCid:PMC9099157
Junaid J., 2011, Breed predispositions to disease in cats and dogs, 2nd edition, Canadian Veterinary Journal-revue Veterinaire Canadienne, 52: 883-883.
Lyons L., 2015, DNA mutations of the cat, Journal of Feline Medicine and Surgery, 17: 203-219.
https://doi.org/10.1177/1098612X15571878
PMid:25701860 PMCid:PMC11148888
Lyons L., Grahn R., Genova F., Beccaglia M., Hopwood J., and Longeri M., 2016, Mucopolysaccharidosis VI in cats-clarification regarding genetic testing, BMC Veterinary Research, 12: 136.
https://doi.org/10.1186/s12917-016-0764-y
PMid:27370326 PMCid:PMC4930586
Matsumoto Y., Ruamrungsri N., Arahori M., Ukawa H., Ohashi K., Lyons L., and Ishihara G., 2020, Genetic relationships and inbreeding levels among geographically distant populations of Felis catus from Japan and the United States, Genomics, 113(1): 104-110.
https://doi.org/10.1016/j.ygeno.2020.11.018
PMid:33246017
Menotti-Raymond M., David V., Pflueger S., Roelke M., Kehler J., O'Brien S., and Narfström K., 2010, Widespread retinal degenerative disease mutation (rdAc) discovered among a large number of popular cat breeds, Veterinary Journal, 186(1): 32-38.
https://doi.org/10.1016/j.tvjl.2009.08.010
PMid:19747862 PMCid:PMC6984347
Michel-Regalado N., Ayala-Valdovinos M., Galindo-García J., Duifhuis-Rivera T., and Virgen-Méndez A., 2022, Prevalence of polycystic kidney disease in Persian and Persian-related cats in western Mexico, Journal of Feline Medicine and Surgery, 24: 1305-1308.
https://doi.org/10.1177/1098612X221114043
PMid:35951480 PMCid:PMC10812362
Ofri R., Reilly C., Maggs D., Fitzgerald P., Shilo-Benjamini Y., Good K., Grahn R., Splawski D., and Lyons L., 2015, Characterization of an early-onset, autosomal recessive, progressive retinal degeneration in bengal cats, Investigative Ophthalmology & Visual Science, 56(9): 5299-5308.
https://doi.org/10.1167/iovs.15-16585
PMid:26258614 PMCid:PMC4539567
O'Neill D., Church D., McGreevy P., Thomson P., and Brodbelt D., 2015, Longevity and mortality of cats attending primary care veterinary practices in England, Journal of Feline Medicine and Surgery, 17: 125-133.
https://doi.org/10.1177/1098612X14536176
PMid:24925771 PMCid:PMC10816413
Pistorius A., and Blokker I., 2021, Statistical analysis in support of maintaining a healthy traditional Siamese cat population. Genetics, Selection, Evolution : GSE, 53: 6.
https://doi.org/10.1186/s12711-020-00596-w
PMid:33407084 PMCid:PMC7789816
Plitman L., Černá P., Farnworth M., Packer R., and Gunn-Moore D., 2019, Motivation of owners to purchase pedigree cats, with specific focus on the acquisition of brachycephalic cats, Animals: an Open Access Journal from MDPI, 9(7): 394.
https://doi.org/10.3390/ani9070394
PMid:31252697 PMCid:PMC6680495
Rokhsar J., Canino J., Raj K., Yuhnke S., Slutsky J., and Giger U., 2021, Web resource on available DNA variant tests for hereditary diseases and genetic predispositions in dogs and cats: an update, Human Genetics, 140: 1505-1515.
https://doi.org/10.1007/s00439-021-02256-5
Samaha G., Beatty J., Wade C., and Haase B., 2019, The burmese cat as a genetic model of type 2 diabetes in humans, Animal Genetics, 50(4): 319-325.
https://doi.org/10.1111/age.12799
PMid:31179570
Si Q.N., 2024, Evolutionary conservation genomics of carnivores: insights from felids, International Journal of Molecular Zoology, 14(3): 154-165.
https://doi.org/10.5376/ijmz.2024.14.0015
Vapalahti K., Virtala A., Joensuu T., Tiira K., Tähtinen J., and Lohi H., 2016, Health and behavioral survey of over 8000 finnish cats, Frontiers in Veterinary Science, 3: 70.
https://doi.org/10.3389/fvets.2016.00070
PMid:27622188 PMCid:PMC5002895
Wang Z.L., and Lin X.F., 2024, Long-term impact of feline calicivirus (FCV): from transmission dynamics to disease management, International Journal of Molecular Veterinary Research, 14(1): 17-22.
http://dx.doi.org/10.5376/ijmvr.2024.14.0003
Associated material
. Readers' comments
Other articles by authors
. Zhaolin Wang
Related articles
. Cat breeding practices
. Hereditary diseases
. Genetic health
. Selective breeding
. Inbreeding
. Genetic testing
. Breed-specific disorders
. Veterinary care
Tools
. Post a comment


