Research Insight
Functional Roles of Key Genes in African Swine Fever Virus Pathogenesis and Immune Evasion 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Veterinary Research, 2024, Vol. 14, No. 4
Received: 24 May, 2024 Accepted: 28 Jun., 2024 Published: 16 Jul., 2024
This study investigated the functional roles of key ASFV genes related to pathogenesis and immune evasion, providing insights into the molecular mechanisms of virus replication, host cell entry, and immune suppression. The main structures and regulatory genes were studied, including p72 (B646L) responsible for capsid formation and host cell entry, as well as the regulatory factor A104R for viral DNA replication. The role of A238L, EP402R (CD2v), and DP71L genes in immune evasion was also analyzed, emphasizing the ASFV induced apoptosis, necrosis, and necrosis pathways leading to tissue damage. A case study of ASFV outbreak in Eastern Europe provides genomic insights into the prevalent strain and evaluates current control measures. The aim of this study is to emphasize potential vaccine targets and strategies for structural protein and gene deletion to reduce the virulence of the virus, promote genomic analysis of African swine fever virus, develop novel antiviral drugs, and collaborate efforts to achieve long-term eradication.
1 Introduction
African swine fever virus (ASFV) is a large, double-stranded DNA virus responsible for African swine fever (ASF), a highly contagious and often fatal disease affecting domestic pigs and wild boars (Wang et al., 2023). The virus primarily targets porcine macrophages, leading to severe hemorrhagic fever with mortality rates approaching 100% in infected swine populations (Zhu et al., 2019; Huang et al., 2023b). ASFV is known for its complex genome, which encodes numerous proteins that facilitate immune evasion and pathogenesis, making it a formidable challenge for disease control and prevention (Dixon et al., 2019; Cheng et al., 2023).
Understanding the pathogenesis of ASFV is crucial due to its devastating impact on the global swine industry, causing significant economic losses and threatening food security (Ye et al., 2023). The virus's ability to evade host immune responses through various mechanisms, such as inhibiting interferon production and modulating host immune pathways, complicates vaccine development and disease management (Frączyk et al., 2016; He et al., 2022). Identifying the key genes involved in ASFV's virulence and immune evasion strategies is essential for developing effective vaccines and therapeutic interventions (Bao et al., 2021; Liu et al., 2023).
This study identified potential targets for vaccine development and antiviral strategies by focusing on the genetic variations and molecular mechanisms of ASFV in regulating host immune responses. It explored the interaction between the encoded proteins of African swine fever virus and host immune pathways, and gained a deeper understanding of the virus's adaptive progress and its impact on controlling African swine fever outbreaks. This study aims to elucidate the functional roles of key ASFV genes in pathogenesis and immune evasion.
2 Key Genes Involved in ASFV Pathogenesis
2.1 p72: capsid protein and viral assembly
The p72 protein is a major capsid protein of the African swine fever virus (ASFV) and plays a crucial role in viral assembly (Figure 1). It is the most abundant structural protein and is essential for the formation of the icosahedral capsid, which is a critical component of the virus's ability to infect host cells. The p72 gene has been sequenced in various ASFV strains, revealing high conservation across different isolates, which underscores its importance in the virus's life cycle. The protein's immunogenic properties make it a target for vaccine development, as it can elicit a protective immune response in the host (Tran et al., 2023).
 Figure 1 ASFV protein (Adapted from Li et al., 2022) Image caption: The distribution of proteins marked with an asterisk (*) was inferred from the predicted or known roles; the genes marked in red are nonessential genes (Adapted from Li et al., 2022) |
2.2 B646L (p72): mediator of host cell entry
The B646L gene, which encodes the p72 protein, is also implicated in mediating the entry of ASFV into host cells. This process is vital for the virus's ability to establish infection. The p72 protein interacts with host cell receptors, facilitating the virus's attachment and subsequent entry into the cell. This interaction is a key step in the viral infection process, as it determines the virus's ability to invade and replicate within the host. The high degree of conservation of the B646L gene across different ASFV strains suggests that it is a critical determinant of the virus's infectivity and pathogenicity (Tran et al., 2023).
2.3 A104R: regulation of viral DNA replication
The A104R gene is involved in the regulation of ASFV DNA replication. This gene encodes a protein that plays a role in the replication machinery of the virus, ensuring the efficient duplication of the viral genome within the host cell. The regulation of DNA replication is crucial for the virus's ability to proliferate and spread within the host. Although the specific mechanisms by which A104R influences DNA replication are not fully understood, its role is considered essential for maintaining the virus's replication fidelity and overall virulence. Understanding the function of A104R could provide insights into potential targets for antiviral strategies aimed at disrupting the replication process of ASFV (Gallardo et al., 2018; Li et al., 2024; Wang et al., 2024).
3 Genes Associated with Immune Evasion
3.1 A238L: NF-κB inhibitor
The A238L gene of the African swine fever virus (ASFV) plays a crucial role in immune evasion by inhibiting the NF-κB signaling pathway (Liu et al., 2024). This gene encodes a protein that interferes with the activation of NF-κB, a key transcription factor involved in the expression of pro-inflammatory cytokines and immune responses (Figure 2). By inhibiting NF-κB, A238L effectively suppresses the host's immune response, allowing the virus to evade detection and destruction by the host's immune system (Gallardo et al., 2018). This mechanism is vital for the virus's ability to establish infection and persist within the host (Huang et al., 2023b).
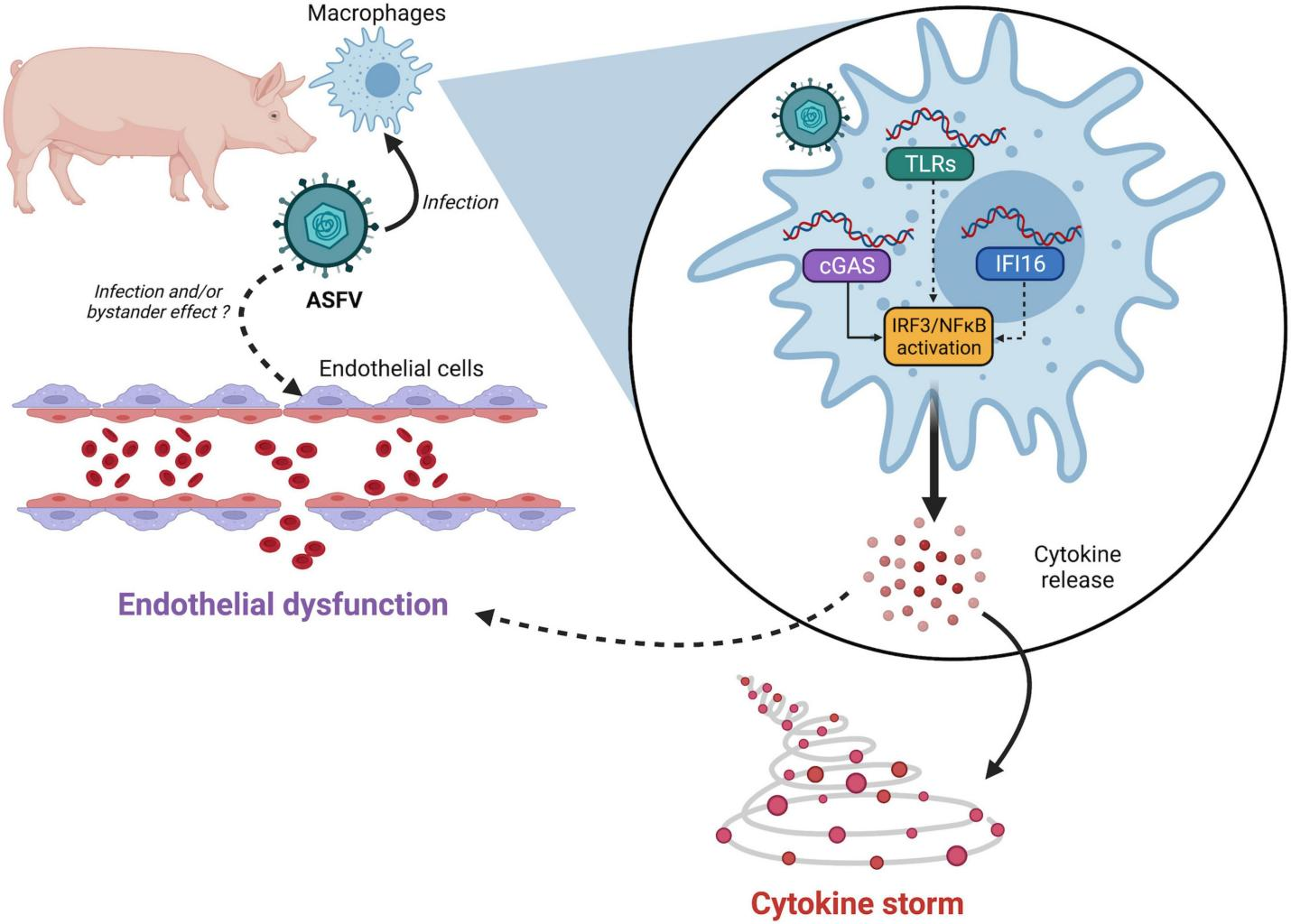 Figure 2 Links between African swine fever virus (ASFV) sensing and pathogenesis (Adopted from Ayanwale et al., 2022) Image caption: ASFV nucleic acids-either incoming DNA or newly synthesized viral DNA/RNA-may be detected by several pattern recognition receptors (PRRs). While cGAS has been validated by multiple teams, the involvement of other PRRs like IFI16 or TLRs cannot be ruled out. ASFV detection then triggers IRF3 and NF-κB activation resulting in uncontrolled secretion of proinflammatory cytokines. Whether endothelial dysfunction is mediated by apoptosis of infected cells or through bystander effects remains to be determined (Adopted from Ayanwale et al., 2022) |
3.2 EP402R (CD2v): inhibition of immune cell adhesion
The EP402R gene, also known as CD2v, is another significant factor in ASFV's immune evasion strategy. This gene encodes a protein that modulates immune cell adhesion, thereby disrupting the normal immune surveillance and response. The EP402R protein affects the hemadsorption phenomenon, which is crucial for the virus's ability to evade the host's immune defenses by altering the interaction between immune cells and infected cells (Bao et al., 2021). This modulation of immune cell adhesion is a strategic mechanism that ASFV uses to prevent effective immune responses and facilitate viral spread within the host (Zhu et al., 2019).
3.3 DP71L: modulation of host stress responses
The DP71L gene is involved in modulating host stress responses, contributing to ASFV's ability to evade the immune system. This gene encodes a protein that interacts with host cellular pathways to modulate stress responses, which can lead to the suppression of apoptosis and other immune defense mechanisms. By altering these pathways, DP71L helps the virus to maintain a favorable environment for replication and persistence within the host (He et al., 2022). This modulation of host stress responses is a critical aspect of ASFV's strategy to evade immune detection and enhance its pathogenicity (Dixon et al., 2019).
4 Mechanisms of ASFV-Mediated Cell Death and Tissue Damage
4.1 Apoptotic pathways induced by ASFV genes
African swine fever virus (ASFV) employs various strategies to induce apoptosis in host cells, primarily through the modulation of host cytokines and specific viral genes (Bosch-Camós et al., 2021). The CD2v and MGF360-505R genes have been shown to influence apoptosis by inhibiting the NF-κB signaling pathway and reducing IL-1β production, thereby decreasing apoptosis levels in porcine alveolar macrophages (PAMs) (Gao et al., 2021; Zhu et al., 2023). Additionally, ASFV genes such as A179L suppress apoptosis by interacting with pro-apoptotic Bcl-2 family proteins, thereby preventing the activation of apoptotic pathways (Shi et al., 2021). The virus also modulates the expression of cytokines like TNF, which are known to induce apoptosis, further contributing to tissue damage.
4.2 Necroptosis and pyroptosis as alternative cell death mechanisms
ASFV not only induces apoptosis but also enhances necroptosis, a form of programmed cell death distinct from apoptosis. The A179L gene of ASFV has been found to enhance necroptosis by promoting the phosphorylation of key proteins such as RIPK1, RIPK3, and MLKL, which are crucial for necroptotic signaling. This shift from apoptosis to necroptosis may serve as an alternative mechanism for the virus to induce cell death and evade host immune responses. While pyroptosis, another form of inflammatory cell death, is not explicitly detailed in the provided data, the modulation of pro-inflammatory cytokines by ASFV suggests potential involvement in pyroptotic pathways (Zhu et al., 2019).
4.3 Cytopathic effects of ASFV on host tissues
ASFV infection leads to significant cytopathic effects in host tissues, primarily through the induction of cell death pathways and immune evasion strategies. The virus targets macrophages and monocytes, leading to excessive inflammatory responses and tissue damage. The deletion of specific ASFV genes, such as those involved in interferon signaling, can attenuate the virus and reduce its cytopathic effects, highlighting the role of these genes in pathogenesis (Sun et al., 2024). The modulation of host immune responses by ASFV, including the suppression of type I interferon production, further exacerbates tissue damage and contributes to the high mortality rates observed in infected swine (Huang et al., 2023a).
5 Case Study: ASFV Outbreak Analysis in Eastern Europe
5.1 Background and scope of outbreak
The African Swine Fever Virus (ASFV) outbreak in Eastern Europe from 2018 to 2023 has been marked by significant challenges in controlling the spread of the virus among swine populations (Figure 3). The outbreak dynamics have been influenced by the genetic variability of ASFV, with notable genetic variants such as those in the EP402R and MGF505-2R genes playing a crucial role in the virus's ability to evade the host immune response and facilitate its spread (Frączyk et al., 2016). The economic and agricultural impact has been severe, with substantial losses in swine populations and disruptions in the pork industry, exacerbating the need for effective control measures (Chen et al., 2024).
 Figure 3 ASF epidemic (Adopted from Li et al., 2022) Image caption: (a) Global ASF epidemic situation from 2015 to 2020; (b) prevalence of ASF in Asia from July to December 2020 (Adopted from Li et al., 2022) |
5.2 Genomic analysis of outbreak strains
Genomic analysis of ASFV strains circulating during the outbreak has identified several virulent gene variants, including those involved in immune evasion such as the A238L and EP153R genes (Gallardo et al., 2018). These variants have been compared with historical ASFV isolates, revealing a slow molecular evolution that suggests a common origin with strains like Georgia 2007/1 and Odintsovo 02/14. Insights into the evolution of immune evasion genes highlight the virus's ability to modulate host immune responses, such as inhibiting interferon production and altering cytokine expression, which are critical for its pathogenesis (Zhu et al., 2019; Ayanwale et al., 2022).
5.3 Control measures and future prevention strategies
Efforts to control the ASFV outbreak have included vaccination strategies targeting immune evasion genes, although the development of effective vaccines remains challenging due to the virus's complex genome and immune evasion capabilities (Li et al., 2022). Biosecurity measures and containment strategies have been implemented to prevent further spread, focusing on controlling movement and improving farm hygiene. Genetic surveillance plays a vital role in outbreak prevention, enabling the tracking of viral evolution and the identification of emerging virulent strains, which is essential for developing targeted interventions (Dixon et al., 2019).
6 Potential Targets for ASFV Vaccine Development
6.1 Key structural proteins as vaccine candidates
The African swine fever virus (ASFV) encodes several structural proteins that play crucial roles in its pathogenesis and immune evasion, making them potential targets for vaccine development. For instance, the CD2v protein, also known as EP402R, is a serotype-specific protein involved in immune modulation and has been considered in vaccine strategies (Sereda et al., 2023). Additionally, the deletion of genes such as EP153R and EP402R has been shown to reduce viral persistence and virulence, suggesting their potential as targets for vaccine development (Petrovan et al., 2021).
6.2 Gene deletion strategies to attenuate ASFV
Gene deletion strategies have been employed to create live-attenuated vaccine candidates by targeting specific virulence-associated genes. For example, the deletion of the H240R gene enhances inflammatory responses and reduces ASFV pathogenicity, providing a basis for developing attenuated vaccines (Huang et al., 2023). Similarly, the deletion of MGF360 and MGF505 genes has resulted in attenuated ASFV strains that confer protection against virulent parental virus challenges (O'Donnell et al., 2015a). Another approach involves the deletion of the 9GL gene, which has been shown to attenuate the virus and induce protective immunity in swine (O'Donnell et al., 2015b).
6.3 Immunomodulatory approaches for ASFV control
ASFV employs various mechanisms to evade the host immune response, which can be targeted for vaccine development. The virus modulates host immune responses by inhibiting MHC Class II antigen processing, suppressing macrophage activation, and inducing immune-suppressive cytokines (Zhu et al., 2019). By understanding these mechanisms, vaccines can be designed to enhance the host's immune response against ASFV. For instance, the deletion of genes involved in immune evasion, such as those regulating NF-κB signaling, can lead to enhanced immune responses and provide a foundation for vaccine development (Gallardo et al., 2018).
7 Future Directions in ASFV Research
7.1 Advances in ASFV genomic and transcriptomic analysis
Recent studies have highlighted the importance of genomic and transcriptomic analyses in understanding the pathogenesis and immune evasion strategies of African swine fever virus (ASFV) (Lin, 2024). Transcriptome analysis has revealed significant gene expression changes in infected swine macrophages, identifying key cytokines and immune pathways involved in ASFV pathogenesis (Zhu et al., 2019). Additionally, genetic variability studies have shown that ASFV genes related to immune evasion, such as those involved in interferon modulation, exhibit notable genetic diversity, which is crucial for tracing the molecular evolution of ASFV isolates (Frączyk et al., 2016). These insights underscore the need for continued genomic and transcriptomic research to identify potential targets for therapeutic intervention and vaccine development.
7.2 Development of novel antivirals targeting ASFV genes
The identification of ASFV genes that play critical roles in immune evasion and pathogenesis presents opportunities for the development of novel antiviral strategies. For instance, the deletion of specific virulence-related genes, such as I73R and H240R, has been shown to attenuate the virus and enhance immune responses, suggesting their potential as targets for live-attenuated vaccines (Liu et al., 2023). Furthermore, understanding the mechanisms by which ASFV proteins, like L83L, inhibit host immune pathways can inform the design of drugs that disrupt these interactions, thereby enhancing host antiviral responses (Cheng et al., 2023). Continued research into the molecular functions of ASFV genes will be essential for the development of effective antiviral therapies.
7.3 Long-term goals for ASFV eradication
The ultimate goal of ASFV research is the eradication of the virus from affected regions. Achieving this requires a multifaceted approach, including the development of effective vaccines and antiviral drugs, as well as improved biosecurity measures (Li and He, 2024). The identification of genetic factors involved in ASFV's adaptive flexibility and host interactions provides a foundation for designing vaccines that can confer broad protection against diverse ASFV strains (Bao et al., 2021). Additionally, the integration of genomic data with epidemiological studies can aid in tracking virus spread and implementing targeted control measures. Long-term eradication efforts will depend on sustained research investment and international collaboration to address the challenges posed by ASFV.
8 Concluding Remarks
African swine fever virus (ASFV) is a complex pathogen that employs various strategies to evade the host's immune system and enhance its pathogenicity. Key insights into ASFV pathogenesis reveal that the virus primarily targets macrophages and monocytes, leading to severe immune modulation. ASFV achieves immune evasion by inhibiting critical immune pathways such as the MHC Class II antigen processing, CD8+ T cell recruitment, and macrophage activation, while also inducing immune-suppressive cytokines. The virus encodes several proteins, such as A238L and H240R, which interfere with interferon production and NF-κB signaling, further aiding in immune evasion. These mechanisms highlight the virus's ability to suppress both innate and adaptive immune responses, contributing to its high virulence and mortality rates in infected swine.
Genomic research plays a pivotal role in understanding ASFV's virulence and immune evasion strategies, which is crucial for developing effective control measures. Studies have identified specific ASFV genes, such as I73R and H240R, that are critical for the virus's pathogenicity and immune evasion, making them potential targets for vaccine development. The identification of genetic variability in ASFV genes related to immune modulation, such as EP402R and MGF505-2R, provides insights into the virus's evolution and potential avenues for tracing outbreaks. By leveraging genomic data, researchers can design live-attenuated vaccines and novel therapeutics that target these virulence factors, offering promising strategies for ASFV control and eradication.
The eradication of ASFV requires a concerted global effort involving collaboration among researchers, governments, and the agricultural industry. Given the virus's rapid spread and devastating impact on the swine industry, it is imperative to enhance surveillance, share genomic data, and develop effective vaccines and antiviral drugs. Collaborative research initiatives can accelerate the identification of novel targets for vaccine development and improve understanding of ASFV's immune evasion mechanisms. By fostering international cooperation and resource sharing, the global community can work towards the successful eradication of ASFV, safeguarding the swine industry and global food security.
Acknowledgments
I am grateful to Miss Zhu for critically reading the manuscript and providing valuable feedback that improved the clarity of the text.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Ayanwale A., Trapp S., Guabiraba R., Caballero I., and Roesch F., 2022, New insights in the interplay between african swine fever virus and innate immunity and its impact on viral pathogenicity, Frontiers in Microbiology, 13: 958307.
https://doi.org/10.3389/fmicb.2022.958307
Bao Y., Qiu J., Luo Y., Rodríguez F., and Qiu H., 2021, The genetic variation landscape of African swine fever virus reveals frequent positive selection and adaptive flexibility, Transboundary and Emerging Diseases, 68(5): 2703-2721.
https://doi.org/10.1111/tbed.14018
PMid:33751854
Bosch-Camós L., López E., Collado J., Navas M., Blanco-Fuertes M., Pina-Pedrero S., Accensi F., Salas M., Mundt E., Nikolin V., and Rodríguez F., 2021, M448R and MGF505-7R: two african swine fever virus antigens commonly recognized by ASFV-Specific T-cells and with protective potential, Vaccines, 9(5): 508.
https://doi.org/10.3390/vaccines9050508
PMid:34069239 PMCid:PMC8156282
Chen S., Wang T., Luo R., Lu Z., Lan J., Sun Y., Fu Q., and Qiu H., 2024, Genetic variations of african swine fever virus: major challenges and prospects, Viruses, 16(6): 913.
https://doi.org/10.3390/v16060913
PMid:38932205 PMCid:PMC11209373
Cheng M., Kanyema M., Sun Y., Zhao W., Lu Y., Wang J., Li X., Shi C., Wang J., Wang N., Yang W., Jiang Y., Huang H., Yang G., Zeng Y., Wang C., and Cao X., 2023, African swine fever virus L83L negatively regulates the cGAS-STING-Mediated IFN-I pathway by recruiting tollip to promote STING autophagic degradation, Journal of Virology, 97(2): e01923-22.
https://doi.org/10.1128/jvi.01923-22
PMid:36779759 PMCid:PMC9973008
Dixon L., Islam M., Nash R., and Reis A., 2019, African swine fever virus evasion of host defences, Virus Research, 266: 25-33.
https://doi.org/10.1016/j.virusres.2019.04.002
PMid:30959069 PMCid:PMC6505686
Frączyk M., Woźniakowski G., Kowalczyk A., Bocian Ł., Kozak E., Niemczuk K., and Pejsak Z., 2016, Evolution of African swine fever virus genes related to evasion of host immune response, Veterinary microbiology, 193: 133-144.
https://doi.org/10.1016/j.vetmic.2016.08.018
PMid:27599940
Gallardo C., Sánchez E., Pérez-Núñez D., Nogal M., De León P., Carrascosa Á., Nieto R., Soler A., Arias M., and Revilla Y., 2018, African swine fever virus (ASFV) protection mediated by NH/P68 and NH/P68 recombinant live-attenuated viruses, Vaccine, 36(19): 2694-2704.
https://doi.org/10.1016/j.vaccine.2018.03.040
PMid:29609966
Gao Q., Yang Y., Quan W., Zheng J., Luo Y., Wang H., Chen X., Huang Z., Chen X., Xu R., Zhang G., and Gong L., 2021, The african swine fever virus with MGF360 and MGF505 deleted reduces the apoptosis of porcine alveolar macrophages by inhibiting the NF-κB signaling pathway and interleukin-1β, Vaccines, 9(11): 1371.
https://doi.org/10.3390/vaccines9111371
PMid:34835302 PMCid:PMC8622997
He W., Yuan J., Ma Y., Zhao C., Yang Z., Zhang Y., Han S., Wan B., and Zhang G., 2022, Modulation of host antiviral innate immunity by african swine fever virus: a review, Animals: an Open Access Journal from MDPI, 12(21): 2935.
https://doi.org/10.3390/ani12212935
PMid:36359059 PMCid:PMC9653632
Huang L., Chen W., Liu H., Xue M., Dong S., Liu X., Feng C., Cao S., Ye G., Zhou Q., Zhang Z., Zheng J., Li J., Zhao D., Wang Z., Sun E., Chen H., Zhang S., Wang X., Zhang X., He X., Guan Y., Bu Z., and Weng C., 2023a, African swine fever virus HLJ/18 CD2v suppresses type I IFN production and IFN-stimulated genes expression through negatively regulating cGMP-AMP synthase-STING and IFN signaling pathways, Journal of Immunology, 210(9): 1338-1350.
https://doi.org/10.4049/jimmunol.2200813
PMid:36971697
Huang L., Liu H., Ye G., Liu X., Chen W., Wang Z., Zhao D., Zhang Z., Feng C., Hu L., Yu H., Zhou S., Zhang X., He X., Zheng J., Bu Z., Li J., and Weng C., 2023b, Deletion of african swine fever virus (ASFV) H240R gene attenuates the virulence of ASFV by enhancing NLRP3-mediated inflammatory responses, Journal of Virology, 97(2): e01227-22.
https://doi.org/10.1128/jvi.01227-22
PMid:36656014 PMCid:PMC9972963
Li J., and He J., 2024, Eliminating porcine pathogens: the role of genetic modifications in enhancing biosafety of transplantable pig organs, Bioscience Methods, 15(4): 162-172.
https://doi.org/10.5376/bm.2024.15.0017
Li M., Liu X., Peng D., Yao M., Wang T., Wang Y., Cao H., Wang Y., Dai J., Luo R., Deng H., Li J., Luo Y., Li Y., Sun Y., Li S., Qiu H., and Li L., 2024, The I7L protein of African swine fever virus is involved in viral pathogenicity by antagonizing the IFN-γ-triggered JAK-STAT signaling pathway through inhibiting the phosphorylation of STAT1, PLOS Pathogens, 20(9): e1012576.
https://doi.org/10.1371/journal.ppat.1012576
PMid:39325821 PMCid:PMC11460700
Li Z., Chen W., Qiu Z., Li Y., Fan J., Wu K., Li X., Zhao M., Ding H., Fan S., and Chen J., 2022, African swine fever virus: a review, Life, 9(5): 103.
https://doi.org/10.3390/life12081255
PMid:36013434 PMCid:PMC9409812
Lin X.F., 2024, Engineering immune-compatible organs: genetic modifications in pigs for reduced rejection in human recipients, Animal Molecular Breeding, 14(1): 106-118.
https://doi.org/10.5376/amb.2024.14.0013
Liu W., Yang L., Di C., Sun J., Liu P., and Liu H., 2024, Nonstructural protein A238L of the african swine fever virus (ASFV) enhances antiviral immune responses by activating the TBK1-IRF3 pathway, Veterinary Sciences, 11(6): 252.
https://doi.org/10.3390/vetsci11060252
PMid:38921999 PMCid:PMC11209439
Liu Y., Shen Z., Xie Z., Song Y., Li Y., Liang R., Gong L., Di D., Liu J., Liu J., Chen Z., Yu W., Lv L., Zhong Q., Liao X., Tian C., Wang R., Song Q., Wang H., Peng G., amd Chen H., 2023, African swine fever virus I73R is a critical virulence-related gene: a potential target for attenuation, Proceedings of the National Academy of Sciences of the United States of America, 120(15): e2210808120.
https://doi.org/10.1073/pnas.2210808120
PMid:37023125 PMCid:PMC10104517
O'Donnell V., Holinka L., Gladue D., Sanford B., Krug P., Lu X., Arzt J., Reese B., Carrillo C., Risatti G., and Borca M., 2015a, African swine fever virus georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus, Journal of Virology, 89: 6048-6056.
https://doi.org/10.1128/JVI.00554-15
PMid:25810553 PMCid:PMC4442422
O'Donnell V., Holinka L., Krug P., Gladue D., Carlson J., Sanford B., Alfano M., Kramer E., Lu Z., Arzt J., Reese B., Carrillo C., Risatti G., and Borca M., 2015b, African swine fever virus georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administered at lowdoses, leads to virus attenuation in swine and induces an effective protection against homologous challenge, Journal of Virology, 89: 8556-8566.
https://doi.org/10.1128/JVI.00969-15
PMid:26063424 PMCid:PMC4524225
Petrovan V., Rathakrishnan A., Islam M., Goatley L., Moffat K., Sánchez-Cordón P., Reis A., and Dixon L., 2021, Role of African swine fever virus (ASFV) proteins EP153R and EP402R in reducing viral persistence and virulence from attenuated BeninΔDP148R, Journal of Virology, 96(1): e01340-21.
https://doi.org/10.1101/2021.04.16.439957
Sereda A., Kazakova A., Namsrayn S., Vlasov M., Sindryakova I., and Kolbasov D., 2023, Subsequent immunization of pigs with african swine fever virus (ASFV) seroimmunotype IV vaccine strain FK-32/135 and by recombinant plasmid DNA containing the CD2v derived from MK-200 ASFV seroimmunotype III strain does not protect from challenge with ASFV seroimmunotype III, Vaccines, 11(5): 1007.
https://doi.org/10.3390/vaccines11051007
PMid:37243111 PMCid:PMC10221915
Shi J., Liu W., Zhang M., Sun J., and Xu X., 2021, The A179L gene of african swine fever virus suppresses virus-induced apoptosis but enhances necroptosis, Viruses, 13(12): 2490.
https://doi.org/10.3390/v13122490
PMid:34960759 PMCid:PMC8708531
Sun H., Yang J., Zhang Z., Wu M., Tian Z., Liu Y., Zhang X., Zhong J., Yang S., Chen Y., Luo J., Guan G., Yin H., and Niu Q., 2024, The african swine fever virus gene MGF_360-4L inhibits interferon signaling by recruiting mitochondrial selective autophagy receptor SQSTM1 degrading MDA5 antagonizing innate immune responses, bioRxiv, 2024-09.
https://doi.org/10.1101/2024.09.09.612163
Tran M., Nguyen H., Le L., Doan H., Nguyen M., Dinh P., and Nguyen B., 2023, Sequencing p72 gene of field strain of African swine fever virus (ASFV) in Vietnam and generation of enhanced immunogenic fusion protein G-p72 potentially expressed as a recombinant antigen in ASFV subunit vaccine, The Journal of Agriculture and Development, 22(6): 32-41.
https://doi.org/10.52997/jad.4.06.2023
Wang T., Luo R., Zhang J., Lan J., Lu Z., Zhai H., Li L., Sun Y., and Qiu H., 2024, The African swine fever virus MGF300-4L protein is associated with viral pathogenicity by promoting the autophagic degradation of IKKβ and increasing the stability of IκBα, Emerging Microbes & Infections, 13(1): 2333381.
https://doi.org/10.1080/22221751.2024.2333381
PMid:38501350 PMCid:PMC11018083
Wang T., Luo R., Zhang J., Lu Z., Li L., Zheng Y., Pan L., Lan J., Zhai H., Huang S., Sun Y., and Qiu H., 2023, The MGF300-2R protein of African swine fever virus is associated with viral pathogenicity by promoting the autophagic degradation of IKKα and IKKβ through the recruitment of TOLLIP, PLOS Pathogens, 19(8): e1011580.
https://doi.org/10.1371/journal.ppat.1011580
PMid:37566637 PMCid:PMC10446188
Ye G., Liu H., Liu X., Chen W., Li J., Zhao D., Wang G., Feng C., Zhang Z., Zhou Q., Zheng J., Bu Z., Weng C., and Huang L., 2023, African swine fever virus H240R protein inhibits the production of type I interferon through disrupting the oligomerization of STING, Journal of Virology, 97(9): e00577-23.
https://doi.org/10.1128/jvi.00577-23
PMid:37199611 PMCid:PMC10537660
Zhu J., Ramanathan P., Bishop E., O'Donnell V., Gladue D., and Borca M., 2019, Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages, PLoS ONE, 14(11): e0223955.
https://doi.org/10.1371/journal.pone.0223955
PMid:31725732 PMCid:PMC6855437
Zhu Z., Li S., Ma C., Yang F., Cao W., Liu H., Chen X., Feng T., Shi Z., Tian H., Zhang K., Chen H., Liu X., and Zheng H., 2023, African swine fever virus E184L protein interacts with innate immune adaptor STING to block IFN production for viral replication and pathogenesis, Journal of Immunology, 210(4): 442-458.
https://doi.org/10.4049/jimmunol.2200357
PMid:36602826

. HTML
Associated material
. Readers' comments
Other articles by authors
. Xiaofang Lin
Related articles
. African swine fever virus
. Pathogenesis
. Immune evasion
. ASFV genes
. Vaccine development
Tools
. Post a comment


