 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Veterinary Research, 2024, Vol. 14, No. 4
Received: 07 Jun., 2024 Accepted: 17 Jul., 2024 Published: 28 Jul., 2024
This study explores the complex relationship between gut microbiota composition and dog immune function, emphasizing the dynamic interaction between microbiota and host defense mechanisms. It deeply analyzes the factors affecting gut microbiota diversity, core microbial species and their functional roles, and compares them with other species. It reviews the mechanisms by which gut microbiota regulates immune responses, including microbial metabolites and immune signaling pathways, and evaluates probiotic, prebiotic, fecal microbiota transplantation, and dietary strategies aimed at supporting dog immune health. A case study on dogs with allergic dermatitis emphasizes the importance of gut microbiota in immune related diseases and demonstrates potential therapeutic pathways. This study identified key knowledge gaps and emphasized the necessity of standardized interventions and personalized methods based on microbiota in veterinary medicine, deepening the understanding of immune interactions between gut microbiota and potentially improving dog health and shaping the future of veterinary care.
1 Introduction
The gut microbiota, a complex community of microorganisms residing in the gastrointestinal tract, plays a crucial role in maintaining canine health. It contributes to host metabolism, protects against pathogens, and educates the immune system, thereby influencing most physiological functions (Suchodolski, 2021). In dogs, the gut microbiome is composed predominantly of five bacterial phyla: Firmicutes, Fusobacteria, Bacteroidetes, Proteobacteria, and Actinobacteria (Pilla and Suchodolski, 2020). The composition of the gut microbiota can be influenced by factors such as age, diet, and environment, and significant alterations, known as dysbiosis, are associated with gastrointestinal dysfunctions and other health issues (Vilson et al., 2018). The gut microbiota's ability to produce metabolites like short-chain fatty acids (SCFAs) and tryptophan derivatives further underscores its role in regulating immune responses and maintaining homeostasis (Ziese and Suchodolski, 2020; Wang et al., 2023).
The immune system is vital for protecting dogs against infections and diseases. It involves a complex network of cells and molecules that work together to identify and eliminate pathogens. The gut is the largest immune organ in the body, housing a significant portion of the immune cells, including macrophages, dendritic cells, and T cells, which interact with the gut microbiota to maintain immune homeostasis (Lee, 2022). The gut microbiota influences the maturation and function of these immune cells, affecting systemic immune responses and potentially contributing to the development of inflammatory and autoimmune diseases (Lo et al., 2020). Understanding the interactions between the gut microbiota and the immune system is essential for developing strategies to enhance canine health and treat immune-related diseases (Yoo et al., 2020).
This study explores the complex relationship between canine gut microbiota and immune response. By examining current research, it elucidates how gut microbiota affects immune function and contributes to canine health, covering the mechanisms by which gut microbiota regulates immune response, the impact of ecological dysbiosis on health, and potential therapeutic interventions such as probiotic and fecal microbiota transplantation. It emphasizes the importance of using canine models to bridge the gap between preclinical studies and human clinical trials, particularly in the context of immunotherapy. Through this comprehensive analysis, this study aims to provide insights for optimizing the gut microbiota to improve the immune health of dogs.
2 Gut Microbiota Composition and Diversity in Dogs
2.1 Factors influencing gut microbiota composition
The composition of the gut microbiota in dogs is influenced by a variety of intrinsic and extrinsic factors. Intrinsic factors include age, physiology, and pathology, while extrinsic factors encompass diet, environment, and medication (Figure 1) (Garrigues et al., 2022). For instance, the gut microbiota of puppies evolves rapidly, with significant changes occurring as they grow from 7 weeks to 18 months of age. This development is influenced by maternal microbiota and living environment, with urban dogs showing higher microbial diversity compared to those in rural areas (Vilson et al., 2018). Additionally, dietary interventions, such as the administration of probiotics, can modulate the gut microbiota composition, as seen with the probiotic mixture Slab51®, which increased beneficial bacteria like Bifidobacterium and Lactobacillus in dogs.
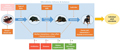 Figure 1 Major factors shaping the development of growing puppies' gut microbiota from birth to adulthood (Adopted from Garrigues et al., 2022) Image caption: Orange boxes show obligate factors, while the pink box illustrates a hypothetical impact. Factors in red boxes are facultative factors that can be associated with dysbiosis, while green boxes are facultative factors with beneficial effects on microbiota balance (Adopted from Garrigues et al., 2022) |
2.2 Core microbial species and their functions
The canine gut microbiota is predominantly composed of five phyla: Firmicutes, Fusobacteria, Bacteroidetes, Proteobacteria, and Actinobacteria. These microbial communities play crucial roles in host metabolism, protection against pathogens, and immune system education. Specific microbial metabolites, such as short-chain fatty acids (SCFAs), are vital for maintaining gut and systemic immune homeostasis by influencing the differentiation and function of immune cells (Wang et al., 2023). The presence of these core microbial species and their metabolic products is essential for maintaining intestinal homeostasis and preventing inflammation (Yoo et al., 2020).
2.3 Comparative analysis with other species
When comparing the gut microbiota of dogs to other species, there are both similarities and differences. Like humans and other animals, dogs have a gut microbiota that contributes to various physiological functions, including metabolism and immune modulation (Rossi et al., 2020). However, the specific composition and diversity of the microbiota can vary significantly. For example, while the predominant phyla in dogs are similar to those found in humans, the relative abundance and specific bacterial species may differ due to factors such as diet and environment (Pilla and Suchodolski, 2020). Additionally, the canine gut microbiota undergoes significant changes during growth and development, which may not be as pronounced in other species.
3 Mechanisms Linking Gut Microbiota to Immune Responses
3.1 Microbiota-immune crosstalk
The gut microbiota and the host immune system engage in a dynamic crosstalk that is crucial for maintaining intestinal homeostasis and regulating immune responses (Yang and Cong, 2021). This interaction involves both innate and adaptive immune systems, where the microbiota influences the development and function of immune cells, and in turn, the immune system shapes the composition of the microbiota (Günther et al., 2016). The gut microbiota produces a variety of metabolites that mediate communication between gut epithelial and immune cells, contributing to the maintenance of the mucosal barrier and reducing intestinal permeability (Yang et al., 2020). Disruptions in this crosstalk can lead to increased susceptibility to infections and inflammatory diseases.
3.2 Microbial metabolites and immune modulation
Microbial metabolites, such as short-chain fatty acids (SCFAs), tryptophan catabolites, and bile acids, play a significant role in modulating immune responses (Zhou, 2024). These metabolites interact with immune cells by binding to specific receptors, influencing the differentiation and function of both immunosuppressive and inflammatory cells. SCFAs, for instance, are known to enhance intestinal epithelial barrier function and promote regulatory immune responses, while tryptophan metabolites can activate the aryl hydrocarbon receptor (AhR), which is crucial for maintaining gut immune homeostasis (Gasaly et al., 2021). The modulation of these pathways by microbial metabolites highlights their potential as therapeutic targets for immune-related diseases (Chénard et al., 2020).
3.3 Disruption of gut microbiota and immune dysregulation
Disruption of the gut microbiota, or dysbiosis, can lead to immune dysregulation and is associated with various diseases, including inflammatory bowel disease, autoimmune disorders, and metabolic conditions (Cheng et al., 2019). Dysbiosis can result in an increased abundance of pathogenic bacteria, which disrupts the epithelial barrier and triggers inflammatory responses (Amoroso et al., 2020). This imbalance can also lead to chronic inflammation and oxidative stress, further exacerbating immune-related diseases (Rizzetto et al., 2018). Therapeutic strategies such as probiotics, prebiotics, and fecal microbiota transplantation are being explored to restore a healthy microbiota composition and improve immune function (Campbell et al., 2023).
4 Clinical Implications and Therapeutic Interventions
4.1 Probiotics and prebiotics in canine health
Probiotics and prebiotics have emerged as significant modulators of the gut microbiota, with potential benefits for canine health (Fang et al., 2019). The administration of probiotic mixtures, such as Slab51®, has been shown to be safe and effective in enhancing immune functions in dogs. This specific probiotic mixture led to a decrease in harmful bacteria like Clostridium perfringens and an increase in beneficial bacteria such as Bifidobacterium and Lactobacillus, alongside elevated levels of fecal IgA and plasma IgG, indicating enhanced immune responses (Rossi et al., 2020). Additionally, probiotics and prebiotics can help ameliorate clinical signs of gastrointestinal diseases by modifying the microbiome composition, even though they may not permanently colonize the gut. These findings suggest that incorporating probiotics and prebiotics into canine diets could support immune health and potentially prevent or manage gastrointestinal disorders.
4.2 Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation (FMT) is gaining attention as a promising intervention for restoring healthy gut microbiota in dogs (Kim et al., 2021). FMT involves transferring fecal matter from a healthy donor to a recipient, aiming to modulate the gut microbiota and improve health outcomes. This method has shown potential in addressing gut dysbiosis, which is linked to various health issues, including behavioral disorders and immune dysfunctions (Kiełbik and Witkowska-Piłaszewicz, 2024). While FMT is still under investigation, it holds promise for treating conditions associated with microbiota imbalances, such as gastrointestinal diseases and possibly even enhancing responses to cancer immunotherapies (Kleber et al., 2022). However, further studies are needed to fully understand its efficacy and limitations in canine health.
4.3 Dietary modifications and immune support
Dietary modifications play a crucial role in supporting the immune system through the modulation of gut microbiota. The composition of the gut microbiome is influenced by diet, which in turn affects immune function and overall health. For instance, specific dietary components can alter the production of microbial metabolites, such as short-chain fatty acids, which are known to have immunomodulatory effects (Campbell et al., 2023). Adjusting the diet to include ingredients that promote a healthy microbiome can help maintain immune homeostasis and prevent dysbiosis-related diseases. Moreover, dietary interventions can be tailored to individual dogs, considering factors like age, health status, and environmental influences, to optimize immune support and overall well-being.
5 Case Study
5.1 Background and study design
Allergic dermatitis in dogs, particularly atopic dermatitis, is a prevalent condition characterized by itchy and inflamed skin. The pathogenesis of this disease is multifactorial, involving skin barrier dysfunction, immunological dysregulation, and alterations in the gut microbiota (Rostaher et al., 2022). A randomized controlled trial was conducted to evaluate the impact of a novel probiotic and nutraceutical supplement on dogs with pruritic dermatitis. This study involved 105 privately owned dogs, with a focus on assessing changes in clinical signs of allergy and gut microbiome composition over a 10-week period. The trial design included a double-blind setup with a placebo group for comparison, ensuring robust data on the effects of the supplement on both the gut microbiota and immune responses.
5.2 Results and key findings
The study found that the probiotic and nutraceutical blend (PNB) led to significant improvements in the clinical signs of pruritic dermatitis compared to the placebo group. Notably, dogs receiving the PNB showed faster resolution of pruritus, with significant differences observed as early as two weeks into the trial (Table 1) (Tate et al., 2024). The gut microbiome analysis revealed an enrichment of beneficial probiotic species and a reduction in pathogenic species in the treated group, suggesting a positive modulation of the gut microbiota. These changes in the gut microbiota were associated with improved immune responses, as evidenced by the reduction in clinical symptoms of dermatitis.
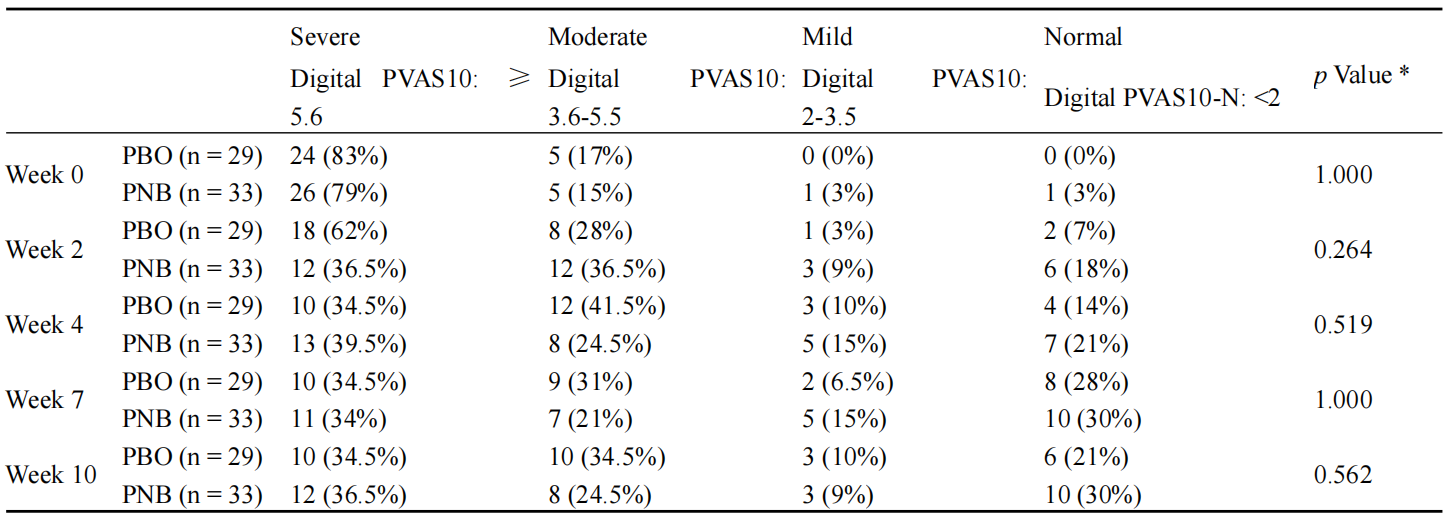 Table 1 Digital PVAS10 severity thresholds (Adopted from Tate et al., 2024) Note: * Fisher’s exact test (Adopted from Tate et al., 2024) |
5.3 Implications for treatment and management
The findings from Craig’s (2016) study underscore the potential of targeting the gut microbiota as a therapeutic strategy for managing allergic dermatitis in dogs. By modulating the gut microbiome, it is possible to influence immune responses and alleviate symptoms of dermatitis. This approach offers a promising adjunct to traditional treatments, which often focus solely on symptomatic relief. The use of dietary supplements containing probiotics and prebiotics could provide a more holistic management strategy, addressing both the underlying immune dysregulation and the gut-skin axis involved in the pathogenesis of allergic dermatitis (Tizard and Jones, 2017). Further research with larger cohorts and more stringent diagnostic criteria is recommended to validate these findings and optimize treatment protocols.
6 Challenges and Future Directions
6.1 Knowledge gaps in canine microbiota research
Despite significant advancements in understanding the canine gut microbiota, there remain substantial knowledge gaps. Current research has primarily focused on the composition of the microbiota in adult dogs, with less emphasis on the developmental stages in puppies. The dynamic process of gut microbiota development in young dogs is influenced by various factors, including maternal and environmental influences, yet the specific mechanisms and long-term health implications remain underexplored (Garrigues et al., 2022). Additionally, while the role of the gut microbiota in immune modulation is recognized, the precise interactions between specific microbial communities and immune responses in dogs are not fully understood (Honneffer et al., 2014). This lack of comprehensive data hinders the ability to develop targeted interventions for enhancing canine health through microbiota manipulation.
6.2 Standardization of microbiota-based interventions
The standardization of microbiota-based interventions, such as probiotics and fecal microbiota transplantation (FMT), presents another challenge. While probiotics have shown potential in modulating the gut microbiota and enhancing immune function, their effects can be inconsistent due to variations in strains, dosages, and administration protocols (Rossi et al., 2020). Moreover, the efficacy of FMT as a treatment for gut dysbiosis in dogs is still under investigation, with further studies needed to establish standardized procedures and evaluate long-term outcomes. The development of a standardized approach is crucial for ensuring the safety and effectiveness of these interventions in clinical settings.
6.3 Potential for personalized canine medicine
The potential for personalized medicine in canine health care is an exciting frontier, driven by the unique microbiota profiles of individual dogs. Personalized approaches could tailor interventions based on specific microbiota compositions and the host's genetic and environmental factors (Wang et al., 2023). However, realizing this potential requires a deeper understanding of the interactions between the microbiota, host genetics, and environmental influences (Zhang, 2024). Additionally, the development of reliable diagnostic tools, such as the "Dysbiosis Index," could facilitate the monitoring of gut health and the customization of treatments. As research progresses, personalized medicine could revolutionize the management of gastrointestinal and immune-related diseases in dogs, offering more precise and effective therapeutic options.
7 Concluding Remarks
The gut microbiota plays a pivotal role in modulating immune responses in dogs, influencing both local and systemic immunity. Studies have shown that probiotic supplementation, such as with Slab51®, can enhance immune function by increasing beneficial bacteria like Bifidobacterium and Lactobacillus, while reducing harmful bacteria such as Clostridium perfringens. This results in elevated levels of fecal IgA and plasma IgG, indicating improved immune responses. Additionally, the gut microbiota's composition is influenced by various factors including age, diet, and environment, which can significantly impact the immune system's development and function. The gut microbiome's metabolites, such as short-chain fatty acids, play a crucial role in maintaining immune homeostasis and preventing dysbiosis-related diseases.
The insights into the gut microbiota's role in immune modulation have significant implications for veterinary practice. Probiotic supplementation can be a valuable tool for enhancing immune function and maintaining gut health in dogs, potentially reducing the incidence of gastrointestinal diseases and improving overall health outcomes. Understanding the factors that influence gut microbiota development, such as maternal and environmental influences, can help veterinarians devise better dietary and management strategies to support optimal microbiota composition and immune function in dogs. The use of tools like the Dysbiosis Index can aid in diagnosing and monitoring gut health, allowing for more targeted interventions.
Future research should focus on further elucidating the complex interactions between gut microbiota and the immune system in dogs. Longitudinal studies examining the effects of different probiotic strains and dietary interventions on gut microbiota and immune responses are needed to optimize therapeutic strategies. Additionally, exploring the potential of fecal microbiota transplantation (FMT) and its long-term effects on gut health and immune function could provide new avenues for treatment. Understanding the genetic and epigenetic impacts of gut microbiota metabolites on immune cells will also be crucial in developing personalized veterinary care approaches.
Acknowledgments
We thank the anonymous reviewers for their insightful comments and suggestions that greatly improved the manuscript.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Amoroso C., Perillo F., Strati F., Fantini M., Caprioli F., and Facciotti F., 2020, The role of gut microbiota biomodulators on mucosal immunity and intestinal inflammation, Cells, 9(5): 1234.
https://doi.org/10.3390/cells9051234
PMid:32429359 PMCid:PMC7291275
Campbell C., Kandalgaonkar M., Golonka R., Yeoh B., Vijay-Kumar M., and Saha P., 2023, Crosstalk between gut microbiota and host immunity: Impact on Inflammation and Immunotherapy, Biomedicines, 11(2): 294.
https://doi.org/10.3390/biomedicines11020294
PMid:36830830 PMCid:PMC9953403
Chénard T., Prévost K., Dubé J., and Massé É., 2020, Immune system modulations by products of the gut microbiota, Vaccines, 8(3): 461.
https://doi.org/10.3390/vaccines8030461
PMid:32825559 PMCid:PMC7565937
Cheng H., Ning M., Chen D., and Ma W., 2019, Interactions between the gut microbiota and the host innate immune response against pathogens, Frontiers in Immunology, 10: 607.
https://doi.org/10.3389/fimmu.2019.00607
PMid:30984184 PMCid:PMC6449424
Craig J., 2016, Atopic dermatitis and the intestinal microbiota in humans and dogs, Veterinary Medicine and Science, 2: 95-105.
https://doi.org/10.1002/vms3.24
PMid:29067183 PMCid:PMC5645856
Fang Z., Lu W., Zhao J., Zhang H., Qian L., Wang Q., and Chen W., 2019, Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: a pilot study, European Journal of Nutrition, 59: 2119-2130.
https://doi.org/10.1007/s00394-019-02061-x
PMid:31342226
Garrigues Q., Apper E., Chastant S., and Mila H., 2022, Gut microbiota development in the growing dog: a dynamic process influenced by maternal, environmental and host factors, Frontiers in Veterinary Science, 9: 964649.
https://doi.org/10.3389/fvets.2022.964649
PMid:36118341 PMCid:PMC9478664
Gasaly N., De Vos P., and Hermoso M., 2021, Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation, Frontiers in Immunology, 12: 658354.
https://doi.org/10.3389/fimmu.2021.658354
PMid:34122415 PMCid:PMC8187770
Günther C., Josenhans C., and Wehkamp J., 2016, Crosstalk between microbiota, pathogens and the innate immune responses, International Journal of Medical Microbiology: IJMM, 306(5): 257-265.
https://doi.org/10.1016/j.ijmm.2016.03.003
PMid:26996809
Honneffer J., Minamoto Y., and Suchodolski J., 2014, Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs, World Journal of Gastroenterology, 20(44): 16489-16497.
https://doi.org/10.3748/wjg.v20.i44.16489
PMid:25469017 PMCid:PMC4248192
Kiełbik P., and Witkowska-Piłaszewicz O., 2024, The relationship between canine behavioral disorders and gut microbiome and future therapeutic perspectives, Animals: an Open Access Journal from MDPI, 14(14): 2048.
https://doi.org/10.3390/ani14142048
PMid:39061510 PMCid:PMC11273744
Kim J., Kim K., and Kim W., 2021, Gut microbiota restoration through fecal microbiota transplantation: a new atopic dermatitis therapy, Experimental & Molecular Medicine, 53: 907-916.
https://doi.org/10.1038/s12276-021-00627-6
PMid:34017060 PMCid:PMC8178377
Kleber K., Iranpur K., Perry L., Cruz S., Razmara A., Culp W., Kent M., Eisen J., Rebhun R., and Canter R., 2022, Using the canine microbiome to bridge translation of cancer immunotherapy from pre-clinical murine models to human clinical trials, Frontiers in Immunology, 13: 983344.
https://doi.org/10.3389/fimmu.2022.983344
PMid:36032113 PMCid:PMC9412231
Lee T., 2022, Gut microbiota and immune responses, Science Insights, 8(10): 1587.
https://doi.org/10.15354/si.22.re022
Lo B., Chen G., Núñez G., and Caruso R., 2020, Gut microbiota and systemic immunity in health and disease, International Immunology, 33(4): 197-209.
https://doi.org/10.1093/intimm/dxaa079
PMid:33367688 PMCid:PMC8011437
Pilla R., and Suchodolski J., 2020, The role of the canine gut microbiome and metabolome in health and gastrointestinal disease, Frontiers in Veterinary Science, 6: 498.
https://doi.org/10.3389/fvets.2019.00498
PMid:31993446 PMCid:PMC6971114
Rizzetto L., Fava F., Tuohy K., and Selmi C., 2018, Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex, Journal of Autoimmunity, 92: 12-34.
https://doi.org/10.1016/j.jaut.2018.05.008
PMid:29861127
Rossi G., Pengo G., Galosi L., Berardi S., Tambella A., Attili A., Gavazza A., Cerquetella M., Jergens A., Guard B., Lidbury J., Stainer J., Crovace A., and Suchodolski J., 2020, Effects of the probiotic mixture Slab51® (SivoMixx®) as food supplement in healthy dogs: evaluation of fecal microbiota, clinical parameters and immune function, Frontiers in Veterinary Science, 7: 613.
https://doi.org/10.3389/fvets.2020.00613
PMid:33102555 PMCid:PMC7499176
Rostaher A., Morsy Y., Favrot C., Unterer S., Schnyder M., Scharl M., and Fischer N., 2022, Comparison of the gut microbiome between atopic and healthy dogs-preliminary data, Animals: an Open Access Journal from MDPI, 12(18): 2377.
https://doi.org/10.3390/ani12182377
PMid:36139237 PMCid:PMC9495170
Suchodolski J., 2021, Analysis of the gut microbiome in dogs and cats, Veterinary Clinical Pathology, 50: 6-17.
https://doi.org/10.1111/vcp.13031
PMid:34514619 PMCid:PMC9292158
Tate D., Tanprasertsuk J., Jones R., Maughan H., Chakrabarti A., Khafipour E., Norton S., Shmalberg J., and Honaker R., 2024, A randomized controlled trial to evaluate the impact of a novel probiotic and nutraceutical supplement on pruritic dermatitis and the gut microbiota in privately owned dogs, Animals : an Open Access Journal from MDPI, 14(3): 453.
https://doi.org/10.3390/ani14030453
PMid:38338095 PMCid:PMC10854619
Tizard I., and Jones S., 2017, The microbiota regulates immunity and immunologic diseases in dogs and cats.. the veterinary clinics of north america, Small Animal Practice, 48(2): 307-322.
https://doi.org/10.1016/j.cvsm.2017.10.008
PMid:29198905
Vilson Å., Ramadan Z., Li Q., Hedhammar Å., Reynolds A., Spears J., Labuda J., Pelker R., Björkstén B., Dicksved J., and Hansson-Hamlin H., 2018, Disentangling factors that shape the gut microbiota in German Shepherd dogs, PLoS ONE, 13(3): e0193507.
https://doi.org/10.1371/journal.pone.0193507
PMid:29570709 PMCid:PMC5865712
Wang J., Zhu N., Su X., Gao Y., and Yang R., 2023, Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis, Cells, 12(5): 793.
https://doi.org/10.3390/cells12050793
PMid:36899929 PMCid:PMC10000530
Yang Q., Wang Y., Jia A., Wang Y., Bi Y., and Liu G., 2020, The crosstalk between gut bacteria and host immunity in intestinal inflammation, Journal of Cellular Physiology, 236: 2239-2254.
https://doi.org/10.1002/jcp.30024
PMid:32853458
Yang W., and Cong Y., 2021, Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases, Cellular & Molecular Immunology, 18: 866-877.
https://doi.org/10.1038/s41423-021-00661-4
PMid:33707689 PMCid:PMC8115644
Yoo J., Groer M., Dutra S., Sarkar A., and McSkimming D., 2020, Gut microbiota and immune system interactions, Microorganisms, 8(10): 1587.
https://doi.org/10.3390/microorganisms8101587
PMid:33076307 PMCid:PMC7602490
Zhang A.T., 2024, Research on the role of DNA methylation in the epigenetic regulation mechanism of Pomeranian dogs, Animal Molecular Breeding, 14(1): 72-81.
https://doi.org/10.5376/amb.2024.14.0009
PMid:39450072
Zhou J.Y., 2024, Microbiome and chronic diseases: association, causal relationship, and therapeutic potential, Molecular Microbiology Research, 14(1): 10-19.
http://dx.doi.org/10.5376/mmr.2024.14.0002
Ziese A., and Suchodolski J., 2020, Impact of changes in gastrointestinal microbiota in canine and feline digestive diseases, the veterinary clinics of north america, Small Animal Practice, 51(1): 155-169.
https://doi.org/10.1016/j.cvsm.2020.09.004
PMid:33131916

. HTML
Associated material
. Readers' comments
Other articles by authors
. Jun Wang
. Jun Li
Related articles
. Canine gut microbiota
. Immune responses
. Probiotics
. Microbiota-immune crosstalk
. Veterinary medicine
Tools
. Post a comment


