Seasonal Variation in Thyroid Gland of the Female Bat, Taphozous Kachhensis (Dobson) During Pregnancy 
2 Department of Zoology, N. H. College, Bramhapuri, India
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Zoology, 2013, Vol. 3, No. 2 doi: 10.5376/ijmz.2013.03.0002
Received: 26 Dec., 2012 Accepted: 09 Jan., 2013 Published: 25 Jan., 2013
Chavhan et al., 2013, Seasonal Variation in Thyroid Gland of the Female Bat, Taphozous Kachhensis (Dobson) During Pregnancy, Intl. J. of Molecular Zoology, Vol.3, No.2, 4-9
The aim of present study was to compare the changes in thyroid gland during the reproductive cycle of the female bat Taphozous kachhensis. The thyroid gland of the bat was studied morphometrically and histologically, it showed marked seasonal variation in weight, quantity of colloid, epithelial cell height and plasma concentration of thyroid hormones. The thyroid gland was a symmetrical, bilobed, and located on the lateral side of the trachea, between the first and the third tracheal rings, connected by an isthmus. The mean weight of the noted lobes was (2.240±0.121) mg, (2.88±0.05) mg and (2.704±0.067) mg, during early pregnancy, mid pregnancy and late pregnancy respectively. The thyroid hormone showed marked seasonal variations. During early pregnancy there was rise in the level of TSH (1.7 ng/mL) but the slight decrease in the level of T3 (54 ng/mL) and T4 (2.9 ng/mL) was noticed. The level of TSH and T4 was decreases during mid pregnancy but the increase in the level of T3 was noticed and it was highest as compare to other stages and it is found to be 1.4 ng/mL, 1.5 ng/mL, 69 ng/mL respectively. During late pregnancy, TSH and T3 concentration decreases but the increase in the level of T4 was observed and it was found to be 0.9 ng/mL, 60 ng/mL, 2.9 ng/mL respectively. The diameter of colloid in small, medium and large follicle during early pregnancy and late pregnancy were 28 µm, 56 µm, 96 µm and 36 µm, 60 µm, 72 µm respectively. The epithelial cell heights of small, medium and large size follicles during early pregnancy and late pregnancy were 3.6 µm, 4 µm, 8 µm and 4 µm, 8 µm, 8 µm respectively.
Introduction
The thyroid gland secretes thyroglobulin, triiodothyronine and thyroxin hormones (Banks, 1993). Thyroxin plays an important role in metabolism of the animal body. Thyroid gland comprises of follicles lined by follicular cells and the interfollicular space was filled with connective tissue (Leeson and Leeson, 1970). The thyroid gland has the ability to concentrate large amount of iodine for the synthesis of thyroxin and thyroid hormones appear to play a key role in the expression of events that underlie seasonal reproductive cycles (Turner, 1966). The thyroid gland has been studied in many seasonally breeding microchiropteran bats by Kwiecinski et al. (1991), Damassa et al. (1995) and these studies indicate that the thyroid increases activity late in the winter or early spring, is active throughout the summer, and regresses by autumn. Such an activity cycle is commonly found in most seasonally breeding mammals.
The hormones secreted by various endocrine glands are directly or indirectly involved in normal functioning of the reproductive processes in various species and the thyroid hormone appear to be vital in reproduction.
Thyroid hormones are unique because they exert effects within almost every tissue of the body. The thyroid hormone deficiencies have resulted in a number of abnormalities with respect to growth, development, behavior, metabolism and reproduction. Schwartz et al. (1992), Shi and Berrel (1992) have reported that the T3 is essential for mammalian reproduction in cattle, the deficiency of which result into female infertility. It has also been reported that a reduction in the secretion and plasma levels of gonadotrophins was associated with hypothyroidism (Hagino, 1971; LaRochelle and Freeman, 1974; Buchanan et al., 1977). Likewise, Dunn et al. (1976) have reported that the daily rhythmic release of luteinizing hormone (LH) and prolactin in rats was altered by thyroidectomy.
Thyroid hormones have a significant mode of action on seasonal reproduction. In 1994, work by researchers in Michigan implicated thyroid hormones as having a role in decreasing LH secretion at the end of the breeding season, resulting in the normal cessation of follicular activity and ovulation. Along with follicle stimulating hormone (FSH), luteinizing hormone (LH) and indirectly, gonadotropin-releasing hormone (GnRH) are hormones responsible for follicle growth and ovulation.
1 Results
1.1 Gross Observations
Gross studies of thyroid gland of female bat Taphozous kachhensis revealed that the gland was located near the first ring and third ring of trachea and consisted of two lobes. An isthmus connected these lobes to each other. The colour of the gland was reddish brown.
1.2 Seasonal Changes in Body, Ovary, and Thyroid Weights
The weights (mean±SEM) of the body, ovary, and thyroid during reproductive phases are shown in Table 1 and Figure 1. There was not much difference in weight of thyroid gland of adult female bats caught during different phases of pregnancy. The thyroid weight showed two peaks coinciding with the peaks of ovary weight during early pregnancy and mid pregnancy. Both thyroid and ovary began to increase in weight from early pregnancy (January to February) and attained a peak during mid pregnancy (Late February to Middle April). Thyroid weights declined during late pregnancy similarly to ovary weight (Figure 1).
Table 1 The mean weights of the body, ovary, and thyroid during different phases of pregnancy |
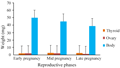 Figure 1 The mean weights of the body, ovary, and thyroid during different phases of pregnancy |
1.3 Histology
The thyroid gland of the female bat is studied histologically; it showed marked seasonal variation in quantity of colloid, epithelial cell height and plasma concentration of thyroid hormones. Diameter of colloid in large, medium and small size follicle of thyroid gland shows variations, with higher value during early pregnancy and lower value during mid pregnancy in large follicle, while in medium size follicle higher value of colloid during late pregnancy and lower during mid pregnancy. Small size follicle shows higher value of colloid during late pregnancy and lower during early pregnancy (Table 2). Thyroid follicular epithelial cells height also showed marked variations during various period of pregnancy. The large size follicles were lined by low cuboidal epithelium having flattened nuclei and 3.6 µm, 4 µm and 4 µm in diameter during early, mid and late pregnancy respectively and were assumed to be inactive. The medium size follicles were lined by cuboidal epithelium having darkly stained nuclei and 4 µm, 8 µm and 8 µm in diameter during early, mid and late pregnancy respectively. The small size follicles were lined by high cuboidal epithelium with rounded nuclei having diameter 12 µm, 12 µm and 8 µm during early, middle and late pregnancy respectively (Figure 2, Table 2, Figure 3).
Table 2 The mean diameter of colloid and epithelial cell height of thyroid during different phases of pregnancy |
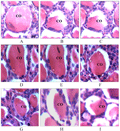 Figure 2 The cuboidal epithelium showed in different stage of pregnancy |
.png) Figure 3 The mean diameter of colloid and epithelial cell height of thyroid during different phases of pregnancy |
1.4 plasma concentration of thyroid hormone
The thyroid hormone examined during different stages of reproductive cycle of bat taphozous kachhensis are described below.
The thyroid hormone shows marked seasonal variations. During early pregnancy there is rise in the level of TSH (1.7 ng/mL) but the slight decrease in the level of T3 (54 ng/mL) and T4 (2.9 ng/mL) was noticed. The level of TSH and T4 was decreases during mid pregnancy but the increase in the level of T3 was noticed and it is highest as compare to other stages and it is found to be 1.4 ng/mL, 1.5 ng/mL, 69 ng/mL respectively. During late pregnancy, TSH and T3 concentration decreases but the increase in the level of T4 was observed and it is found to be 0.9 ng/mL, 60 ng/mL, 2.9 ng/mL respectively (Table 3, Figure 4).
Table 3 Hormonal concentration of Thyroid hormone and TSH during various phases pregnancy |
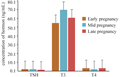 Figure 4 Hormonal concentration of Thyroid hormone and TSH during various phases pregnancy |
2 Discussion
Thyroid gland secretes thyroglobulin, triiodothyronine and thyroxin. Thyroxin plays an important role in metabolism of the body (Turner, 1966). Thyroid gland is metabolically important and essential for the normal maintenance of reproductive function, impairment of thyroid activity may be inhibitory to reproduction (Peebles et al., 1984; Jannini et al., 1995).
Pregnancy alters thyroid status in rodents. In the rat, pregnancy results in decreased total T4 and T3 concentrations and enlarged thyroid gland volume. However, unlike the case of humans, iodine up take is decreased in pregnant rats, and urinary iodide excretion remains unaltered the last days of gestation (Calvo et al., 1990, Feldman, 1958, Versloot et al., 1997).
In Taphozous kachhensis, the location of thyroid gland in the body is similar to other large animals like cattle and buffaloes (Getty et al., 1986) and Camel (Kausar and shahid, 2006) i.e., with the first ring of trachea and consisted of two lobes on both side and an isthmus connecting these lobes. In Taphozous kachhensis the gland appeared reddish brown in colour which is in concordance with the findings of Schwartz and Dioli (1992). The present study also showed a close relationship between changes in the weight and morphological features of the thyroid and the ovarian cycle of Taphozous kachhensis.
Histological studies revealed similar results as reported in camel (Abdel-Magied et al., 2000; Atoji et al., 1999) i.e. the gland consisted of follicles of variable sizes with small size follicle lined by high cuboidal epithelium to columnar epithelial cells, while the larger ones were lined by low cuboidal epithelial cells. Medium size follicles were lined by cuboidal epithelium. Similar observation were reported in Taphozous longimanus (Nerkar, 2007), Megaderma lyra (Sonwane, 2010).
In present study the thyroid hormone show marked seasonal variation. The concentrations of TSH, T3, and T4 show significant seasonal changes. The hormonal level of TSH, T3, T4 changes during early pregnancy and observed 1.7 ng/mL, 54 ng/mL and 2.9 ng/mL respectively. During mid pregnancy the T3 level is found to be 69 ng/mL and 60 ng/mL during late pregnancy. T4 level is highest during early and late pregnancy (2.9 ng/mL) and lowest during mid-pregnancy (1.5 ng/mL). The T3 level is higher than T4 level during reproductive cycle. The serum T3 and T4 concentration showed significant variation with changes in reproductive cycle of bat, Taphozous longimanus (Singh et al., 2005).
The thyroid hormones are important for regulation of nutrient assimilation, metabolism, calorigenesis (Todini et al., 2007), reproduction (Blaszczyk et al., 2004). The report of thyroid hormone on pregnancy was reported in Macrotus californicus (Burn et al., 1972). Thyroid hormone plays an important role in the central regulation of body temperature, stimulating the thermogenesis and regulating cellular metabolism (Seitz et al., 1985). The metabolism hormone Thyroxin (T4) has been implicated in the physiological regulation of energy balance as well as maintaining normal reproductive function in mammals (Bosewell et al., 1994).
In ewes, plasma T4 levels are lower during the luteal phase; T3 concentrations were higher during the luteal phase, this observation supports the present study.
During pregnancy, thyroid activity and circulating hormone levels are reported to increase in all the investigated mammalian species. Several mechanisms have been claimed to explain these observations. Towards the end of pregnancy, the goat foetus (es) should play a competitive role (higher thyroid activity, iodine affinity and uptake than maternal ones), so that a decrease in maternal plasma T4 concentrations has been observed (McDonald et al., 1988).
Plasma T4 concentration was highest during early pregnancy and decreased gradually, reaching lowest values during late pregnancy and post partum (Assane and Sere, 1990; Okab et al., 1993; Yildiz et al., 2005). Like in goats, maternal T3 and T4 in twin pregnancy were lower compared with single-bearing sheep (Yildiz et al., 2005), especially at the end of pregnancy (Assane and Sere, 1990).
3 Material and Methods
All bats were trapped alive from Ambai-Nimbai adjacent to Kampa-Tempa. Body weight of each bat was recorded. Based on the reproductive cycle of Tapozous kachhensis, females were classified into the following three stages:
Early pregnancy (January to February): Ovary shows well developed introvert corpus luteum occupied 3/4 part of ovary.
Middle pregnancy (Late February to Mid April): Ovary shows well developed introvert corpus luteum occupied entire ovary except small peripheral region.
Late pregnancy (Late April to Late May): Ovary shows regress corpus luteum with shrunken luteal cells.
3.1 Collection of Serum and Tissues Histology
The female bats were sacrificed as soon as they arrived in the laboratory. Their blood serum was collected and assayed in laboratory. Ovary and thyroid were excised out from the body cavity and excess fat and connective tissue attached were separated out. All the tissues were fixed in Alcoholic Bouin’s fluid for 24 h, followed by preservation in 70% ethyl alcohol. Each tissue was weighed separately after placement in 70% alcohol. The tissues were dehydrated in ethanol, cleared in xylol, embedded in paraffin wax, serial sections at 6 μm were cut and stained with haematoxylin and eosin.
3.2 Morphometry
Thyroid follicular epithelial height and diameter of colloid was measured using ocular micrometer. Measurements were taken from at least 5 different randomly selected thyroid follicles. The areal fraction of the colloid within a particular section of the thyroid gland was estimated using standard point counting techniques (Weibel, 1979; Singh and Krishna, 1998). The sections used for morphometric analysis were selected by systematic random scheme (West, 1993).
Acknowledgements
I am thankful to Principal Dr. N. S. Kokode for providing us necessary facilities in the laboratory during the course of this work.
Author’s contributions
Dr. Amir A. Dhamani contributed considerably during data collection, analysis of the result and write-up of the manuscript and instrumental during the preparation of this manuscript.
References
Abdel-Magied E. M., Taha A. A. M., and Abdalla A. B., 2000, Light and electron microscopic study of thyroid gland of camel (Camelus dromedaries), Anat histol embryol, 29(6): 331-336
http://dx.doi.org/10.1046/j.1439-0264.2000.00260.x
Assane M., and Sere A., 1990, Seasonal and gestational variations of triiodothyronine and thyroxine plasma-concentrations in Sahel Peulh ewe, Annales de Recherches Veterinaires, 21: 285-289
PMid: 2288455
Atoji Y., Yamamoto Y., Suzuki Y., and Sayed R., 1999, Ultrastructures of the thyroid gland of the one-humped camel (camelus dromedarius), Anat. Histol. Embryol., 28(1): 23-26
http://dx.doi.org/10.1046/j.1439-0264.1999.00152.x
Banks W. J., 1993, Applied veterinary histology, 3rd ed., Mosby-year book, inc. Usa, pp: 414-415
Blaszczyk B., Udala J., and Gaczarzewicz D., 2004, Changes in estradiol, progesterone, melatonin, prolactin and thyroxin concentrations in blood plasma of goats following induced estrus in and outside the natural breeding season, Small Rumin. Res., 51(3): 209-219
http://dx.doi.org/10.1016/S0921-4488(03)00190-1
Bosewell T., Woods S. C., and Kenagy G. J., 1994, Seasonal changes in body mass, insulin and glucocorticoids of free living golden mantled ground squirrels, Gen. Comp. Endocrinol., 96(3): 339-346
http://dx.doi.org/10.1006/gcen.1994.1189
Buchanan G. C., Tredway D. R., Pittard J. C. and Daane T. A, 1977, Gonodotropin secretion and hypothyroidism, Obstet. Gynecol., 50(4): 392-396
PMid: 904799
Burn J. M., Baker R. J. and Bleirer W. J., 1972, Hormonal control of "Delayed development" in Macrotus waterhoushii, I changesin plasma thyroxine during pregnancy and lactation, Gen. Comp. Endocrinol., 18: 54-58
Calvo R., Obregon M. J., OÅ„a C. R. D., Ferreiro B., Rey F. E. D., and Escobar G. M. D., 1990, Thyroid hormone economy in pregnant rats near term: a "physiological" animal model of nonthyroidal illness? Endocrinology, 127(1):10–16
http://dx.doi.org/10.1210/endo-127-1-10
Damassa D. A., Gustafson A. W., Kwiecinski C. G., and Gagin G. A, 1995, Seasonal influences on the Control of plasma sex hormone-binding globulin by T4 In male little brown bats, Am. J. Physiol., 268(5): R1303-R1309
PMid: 7771594
De L. V., la Marca A., Lanzetta D., and Morgante G., 1998, Thyroid function in early pregnancy I: Thyroid-stimulating hormone response to thyrotropin releasing hormone, Gynecological Endocrinology, 12(3): 191-196
http://dx.doi.org/10.3109/09513599809015544
Dunn J. D., Hess M., and Johnson D. C, 1976, Effect of thyroidectomy on rhythmic gonadotropin release, Exp. Biol. Med., 151(1): 22-27
Feldman J. D., 1958, Iodine metabolism in pregnancy, American Journal of Physiology, 192(2): 273-278
PMid: 13508868
Getty R., Sission S., and Grossman J. D., 1986, The anatomy of domestic animals, 5th ed., w. B. Saunders Company, Philadelphia, USA
Glinoer D., 2001, Pregnancy and iodine, Thyroid, 11(5): 471-481
http://dx.doi.org/10.1089/105072501300176426
Hagino N., 1971, Influence of hypothyroid state on ovulation in rats, Endocrinology, 88(6): 1332-1336
http://dx.doi.org/10.1210/endo-88-6-1332
Jannini E. A., Ulisse S., and D'armiento M., 1995, Thyroid hormone and male gonadal function, Endocrine Ver., 16(4): 443-459
Kausar R., and Shahid R.U., 2006, Gross and microscopic anatomy of Thyroid gland of one-humped camel (camelus dromedaries), Pakistan Vet journal, 26(2): 88-90
Kwiecinski G. G., Damassa D. A., and Gustafson A. W, 1991, Patterns of plasma sex hormone-binding globulin, thyroxine and thyroxine-binding globulin in relation to reproductive state and hibernation in female little brown bats, Journal of endocrinology, 128, 63-70
http://dx.doi.org/10.1677/joe.0.1280063
Larochelle F. T., and Freeman M. E, 1974, Superimposition of thyroid hormone regulation on gonadotropin Secretion, Endocrinology, 95(2): 379-387
http://dx.doi.org/10.1210/endo-95-2-379
Leeson T. S., and Leeson C. R., 1970, Histology, 2nd ed., W. B. Saunders Company, Philadelphia, USA
PMCid: 1701701
McDonald B. J., Stocks D. C., Connell J. A., and Hoey W. A., 1988, Thyroxine concentration in maternal and foetal plasma during pregnancy in Australian feral goats, Journal of Agricultural Science, Cambridge, 110: 25–30
http://dx.doi.org/10.1017/S0021859600079648
Nerkar A. A., 2007, Electron microscopic studies on the endocrine gland and reproductive organs of emballonurid female bat Taphozous longimanus (Hardwicke) during reproductive cycle, Ph.D. Thesis submitted to Rashtra Sant Tukdoji Maharaj, Nagpur University, Nagpur, Maharashtra, India
Okab A. B., Elebanna I. M., Mekkawy M. Y., Hassan G. A., Elnouty F. D. EI., and Salem M. H., 1993, Seasonal-changes in plasma thyroid-hormones, total lipids, cholesteroland serum transaminases during pregnancy and at parturition in barki and rahmani ewes, Indian Journal of Animal Sciences, 63(9): 946-951
Peebles E. D., Painter J. A., and Bradley E. L,1984, A possible role for the thyroid in reproductive inhibition in laboratory populations of the prairie deermouse(Peromyscus maniculatus), Comp. Biocherm. Physiol., 77(9): 293-298
http://dx.doi.org/10.1016/0300-9629(84)90063-X
Schwartz H. J., and M. Dioli, 1992, The one-humped camel in eastern Africa, A pictorial guide to diseases, health care and management, Schonwald druck, Berlin. F.r. Germany, pp. 228-229
Seitz H. J., Muller M. J., and Soboll S., 1985, Rapid thyroid-hormone effect on mitochondrial and cytosolic ATP / ADP ratios in the intact liver cell, Biochem. J., 227(1): 149-153
PMid: 3994679
Shi Z. D., and Barrell G. K., 1992, Effects of thyroidectomy on seasonal patterns of live weight, testicular function, antler development and moting in red deer stage, In. Ed. R.D. Brown (ed.) Proc. 2nd Int. Symp. Biol. Deer, Springer Verlag, New York
Singh K., and Krishna A., 1998, Seasonal changes in circulating Serum concentration and in vitro testicular secretion of testosterone and androstenedione in the male Vespertilionid bat, Scotophilus heathi. J. Exp. Zool., 276(1): 43-52
http://dx.doi.org/10.1002/(SICI)1097-010X(19960901)276:1<43::AID-JEZ5>3.0.CO;2-5
Singh U. P., Krishna A., and Bhatnagar K. P., 2005, Seasonal changes in thyroid activity in the female sheath tailed bat, Taphozous longimanus (chiroptera: Emballonuridae), Acta biologica hungarica, 53(3) : 267-278
http://dx.doi.org/10.1556/ABiol.53.2002.3.3
Sonwane D. P., 2010, Endocrine regulation of reproduction in the Indian female vampire bat Megaderma lyra lyra (Geoffroy), Ph.D. Thesis submitted to Rashtra Sant Tukdoji Maharaj, Nagpur University, Nagpur, Maharashtra, India
Todini L., Malfatti A., Valbonesi A., Trabalza-Marinucci M., and Debenedetti A., 2007, Plasma total T3 and T4 concentrations in goats at different physiological stages, as affected by the energy intake, Small Ruminant Research, 68(3), 285-290
http://dx.doi.org/10.1016/j.smallrumres.2005.11.018
Turner C. D., 1966, General endocrinology, 4th ed., W. B. Saunders Company, London, UK
Vasudevan N., Ogawa S., and Pfaff D., 2002, Estrogen and thyroid hormone receptor interactions: physiological flexibility by molecular specificity, Physiol Rev, 82(4): 923-944
PMid: 12270948
Versloot P. M., Schr?der-van Der Elst J. P., van Der Heide D., and Boogerd L, 1997, Effects of marginal iodine deficiency during pregnancy: iodide uptake by the maternal and fetal thyroid, Am J Physiol, 273(6): E1121-E1126
PMid: 9435527
Weibel E. R, 1979, Stereological method, vol. 1, Practical Method for biological morphometry, New York, academic Press
West M. J, 1993, New stereological methods for counting Neurons, Neurobiol. Aging, 14(4): 275-285
http://dx.doi.org/10.1016/0197-4580(93)90112-O
Yildiz A., Balikci E., and Gurdogan F., 2005, Changes in some serum hormonal proï¬les during pregnancy in single- and twin foetus-bearing Akkaraman sheep, Medycyna Weterynaryjna, 61(10): 1138-1141
. PDF(265KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Pankaj Chavhan
. Amir Dhamani
Related articles
. Bat ( Taphozous kachhensis )
. Gross anatomy
. Histology
. Thyroid gland
. T3
. T4
Tools
. Email to a friend
. Post a comment


