Research Article
Description a New Species of Genus Kurixalus (Amphibia: Anura: Rhacophoridae) from Chengdu Prefecture, Sichuan Province, China 
2 State Key Laboratory of Tree Genetics and Breeding, Key Laboratory of Tree Breeding and Cultivation of the National Forestry and Grassland Administration, Research Institute of Forestry, Chinese Academy of Forestry, Beijing, 100091, China
3 Beauty of Science, Anhui Xinzhi Digital Media Information Technology Co., Ltd, Hefei, 230088, China
4 Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, 650223, China
5 Institute of herpetology, Shenyang Normal University, Shenyang, 110034, China
6 Zoological Institute, Russian Academy of Science, St Petersburg State University, 199034, Russia
* These authors contributed equally to this work
http://zoobank.org/References/92E1C1DA-F6F4-4CCB-BEBC-E6D8AA63DBE8
 Author
Author  Correspondence author
Correspondence author
Animal Molecular Breeding, 2021, Vol. 11, No. 2 doi: 10.5376/amb.2021.11.0002
Received: 20 Aug., 2021 Accepted: 13 Dec., 2021 Published: 27 Dec., 2021
Hou M., Peng X.P., Miao J.L., Liu S., Li P.P., and Orlov N.L, 2021, Description a new species of genus Kurixalus (Amphibia: Anura: Rhacophoridae) from Chengdu Prefecture, Sichuan Province, China, Animal Molecular Breeding, 11(2): 1-16 (doi: 10.5376/amb.2021.11.0002)
Eight specimens of frilled swamp tree frogs of Genus Kurixalus were collected from Pingle Town, Qionglai County, Chengdu Prefecture, Sichuan Province, China in April to May 2020. Based on morphological and molecular evidence, we describe these specimens as a new species of Kurixalus. Kurixalus silvaenaias sp. Nov., which are similar to K. idiootocus in appearance and phylogenetically are sister to each other, but morphologically the former can be distinguished from K. idiootocus and other congeners by the following combination of characters: body size 29.6-32.9 mm in males; single internal vocal sac; snout obtuse and rounded with no obvious dermal prominence on tip; vomerine teeth present; nuptial pads less developed, present on the base of first finger in males; throat coarsely granulated; tuberculated dorsal and lateral skin; outer metacarpal tubercles present; dorsal surface yellowish brown with a large hourglass shaped dark brown marking and without any green speckles; pair of large symmetrical dark blotches on chest; and abdomen dark purple. The new species is the first record of Kurixalus in Sichuan, China.
Introduction
Frogs of genus Kurixalus Ye, Fei, and Dubois, 1999 (Frilled Swamp Treefrogs) are distributed from east to south Asia, include the countries of Cambodia, China, India, Indonesia, Japan, Laos, Malaysia, Myanmar, Philippine, Thailand and Vietnam. Now this genus contains 20 species including K. absconditus Mediyansyah, Hamidy, Munir, and Matsui, 2019, K. appendiculatus (Günther, 1858), K. baliogaster (Inger et al., 1999), K. banaensis (Bourret, 1939), K. berylliniris Wu, Huang, Tsai, Li, Jhang, and Wu, 2016, K. bisacculus (Taylor, 1962), K. chaseni (Smith, 1924), K. eiffingeri (Boettger, 1895), K. gracilloides (Nguyen et al., 2020), K. hainanus (Zhao et al., 2005), K. idiootocus (Kuramoto and Wang, 1987), K. lenquanensis (Yu et al., 2017a), K. motokawai (Nguyen et al., 2014b,) K. naso (Annandale, 1912), K. odontotarsus (Ye et al., 1993), K. raoi (Zeng et al., 2021), K. verrucosus (Boulenger, 1893), K. viridescens (Nguyen et al., 2014a), K. wangi (Wu et al., 2016), K. yangi (Yu et al., 2018) (Frost, 2021).
Currently a total of 11 species of Kurixalus are known in China, including K. berylliniris, K. eiffingeri, K. hainanus, K. idiootocus, K. lenquanensis, K. naso, K. odontotarsus, K. raoi, K. verrucosus, K. wangi and K. yangi. They are ranged from Taiwan southeastward and westward to Hainan, Guangdong, Guangxi, Guizhou, Yunnan and Tibet (http://www.amphibiachina.org/), but this genus has never been reported from Sichuan, the northern most record of above species is K. naso where from Baibung Town (N29°17′), Medog County, southeast Tibet. And the closed known locality with Chengdu of this genus is K. raoi which distributed in Qingshuihe Nature Reserve (N25°18′), Southwest Guizhou Autonomous Prefecture, Guizhou Province within the basin of Zhujiang River (Zeng et al., 2021). Therefore, if have the species of genus Kurixalus in north area from the north area of known ranges especially from Yangze River Basin that is not only very important to study the original, spread and diversity of this genus, but also a key point to the relationship between mainland China and Taiwan Island.
During the field surveys from April 2020 to July 2021, we collected 8 specimens of Kurixalus from Chengdu Prefecture, Sichuan Province, China. These specimens are similar to K. idiootocus in appearance, but the results of morphological comparisons and molecular analysis indicated these specimens should be referred to a new taxon, thus we describe the new species in detail as below.
1 Materials and Methods
1.1 Sampling
Specimens (No. CIB 118049-118056) (Table 1) were collected from Qionglai County (30°36′ 16″ N, 103°29′ 69″ E, 623 m elevation), Chengdu Prefecture, Sichuan Province, China and stored in Chengdu Institute of Biology, Chinese Academy of Sciences (CIB). Liver or muscle tissues were preserved in 95% alcohol.
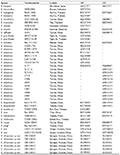 Table 1 Species used in phylogenetic analysis of this study (B. = Buergeria, K. = Kurixalus) |
1.2 DNA sequencing
Genomic DNA was extracted from muscle tissue fixed in 99% ethanol. Tissue samples were digested using proteinase K, and subsequently purified following a standard phenol/chloroform isolation and ethanol precipitation. Fragments encoding mitochondrial 16S rRNA and COI genes were amplified and sequenced following the protocols of Du et al. (2020) and Zeng et al. (2021). Homologous sequences of other Kurixalus species were obtained from GenBank and all new sequences were deposited in GenBank under Accession Nos. OL898656-OL898661.
1.3 Molecular analyses
Sequences were aligned in ClustalX v1.83 (Thompson et al., 1997) with the default parameters; the best substitution model was selected using Modeltest v3.7 (Posada and Crandall, 1998). Phylogenetic relationship was constructed using Bayesian inference (BI) in MrBayes v3.1.2 (Huelsenbeck and Ronquist, 2001). Two runs were performed simultaneously with four Markov chains starting from random trees. The Markov chains were run for 5,000,000 generations and sampled every 100 generations. Generations sampled before the chain reached stationarity were discarded as burn-in, and the remaining trees were used to create a 50% majority-rule consensus tree and to estimate Bayesian posterior probabilities (BPPs).
1.4 Morphology
All measurements were taken with digital calipers to the nearest 0.01 mm and rounded to 0.1 mm. The following abbreviations were used:
Body and head: SVL, snout-vent length; AG, axilla to groin, distance from posterior base of forelimb to anterior base of hindlimb; HL, head length from the rear of the lower jaw to the tip of the snout; HW, head width at the greatest cranial width; HD, head depth, greatest transverse depth of head, taken beyond interorbital region; FIOD, fore-interorbital distance; HIOD, hind-interorbital distance; ED, eye diameter; UEL, upper eyelid length: greatest diameter of upper eyelid; SED, upper eyelid depth: greatest depth of upper eyelid; OL, orbital length: the greatest diameter of orbital; OD, orbital depth: the greatest depth of supra eyelid; TDH, horizontal diameter of tympanum; TDV, Vertical diameter of tympanum; ESL, tip of snout-eye distance; TED, tympanum-eye distance from anterior edge of tympanum to posterior corner of the eye; IND, internarial distance: distance between nostrils; END, eye to nostril distance: distance from anterior corner of eye to nostril; MOP, mandible to posterior orbital, distance from the posterior end of mouth to posterior edge of orbital; MOA, mandible to orbital, distance from the posterior end of mouth to anterior edge of orbital; MN, mandible to nostril, distance from the posterior end of mouth to posterior edge of orbital.
Forelimbs: FLL, length of forelimb from tip of disk of finger III to axilla; ABL, Length of antebrachium from wrist to elbow joint; PL, Length of Palm tip of disk of finger III to wrist; FD1, wide of first finger disk, greatest wide of disk on first finger; FD2, wide of first second disk, greatest wide of disk on second finger; FD3, wide of third finger disk, greatest wide of disk on third finger; FD4, wide of forth finger disk, greatest wide of disk on forth finger; FFL1, first finger length; FFL2, second finger length; FFL3, third finger length; FFL4, forth finger length; NPL, nuptial pad length; IMCL, length of inner metacarpal tubercle.
Hindlimbs: HLL, length of hindlimb from tip of disk of toe IV to groin; FL, femur length; TL, tibia length; FOT, length of hindlimb from tip of disk of toe IV to posterior edge of tibia; TD1, wide of first toe disk, greatest wide of disk on first toe; TD2, wide of second toe disk, greatest wide of disk on second toe; TD3, wide of third toe disk, greatest wide of disk on third toe; TD4, wide of forth toe disk, greatest wide of disk on forth toe; TD5, wide of second fifth disk, greatest wide of disk on fifth toe; FTL1, first toe length; FTL2, second toe length; FTL3, third toe length; FTL4, forth toe length; FTL5, fifth toe length; IMTL, length of inner metatarsal tubercle.
Roman numbers refer to the fingers and toes, the Arabic numbers refer to the number of subarticular tubercles and phalanges: I, inner side; E, exterior side of phalanges.
Comparative morphological data of other Kurixalus species were obtained from previous publications (Yu et al., 2017a; Yu et al., 2018; Zeng et al., 2021; Nguyen et al., 2014a; Nguyen et al., 2014b; Nguyen et al., 2020; Wu et al., 2016; Inger et al., 1999; Taylor, 1962; Mediyansyah et al., 2019; Zhao et al., 2005).
2 Results
The obtained alignments of 16S and COI sequences were 872 bp and 807 bp, respectively. The GTR + I + G model was selected as the best-fit for the combined data of the two genes. Bayesian phylogenetic analysis of the two genes strongly supported Kurixalus specimens from Sichuan as a distinct lineage and sister to K. idiootocus (Figure 1). The genetic divergence between the new lineage and K. idiootocus were 2.3% and 4.6% for 16S and COI, respectively (Table 2).
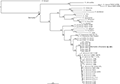 Figure 1 Bayesian phylogram of Kurixalus inferered from 16S and COI sequences. Numbers above branches are Bayesian posterior probabilities (BPP) |
 Table 2 Uncorrected pairwise distances (%) between members of Kurixalus estimated from 16S rRNA (lower triangle) and COI sequences (upper triangle). NA: not available |
Morphological measurements of the Sichuan specimens are given in Table 3. These specimens can be distinguished from all other known congeners by a combination of series characters, e.g. body size 29.6-32.9 mm in males, single internal vocal sac, presence of vomerine teeth, coarsely granulated throat, pair of large symmetrical dark blotches on chest, and dorsal surface yellowish brown with a large hourglass shaped dark brown marking with wide black edge. Therefore, based on molecular and morphological evidence, we describe the lineage consisting of Sichuan specimens as a new species of Kurixalus below.
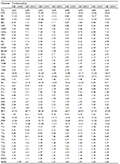 Table 3 Measurements (in mm) of Kurixalus silvaenaias sp. nov. |
2.1 Taxonomy
Kurixalus silvaenaias sp. nov.
http://zoobank.org/NomenclaturalActs/18C6F79F-72DF-4E4A-9789-B374EA50E9A3
Holotype: CIB 118049. (Figure 2)
 Figure 2 Holotype specimen of Kurixalus silvaenaias (CIB 118049) Note: Photographs by Guo-hua YU |
Paratypes: CIB 118050-118056. (Figure 3)
 Figure 3 Paratype specimens of Kurixalus silvaenaias (CIB 118050-118056) Note: Photographs by Guo-hua YU |
Type locality: Pingle Town (30°36′ 16″ N, 103°29′ 69″ E, 623 m elevation), Qionglai County, Chengdu Prefecture, Sichuan Province, China.
Suggest English name: Fairy Swamp Treefrog.
Suggest Chinese name: 精灵原指树蛙 (Jīng Líng Yuán Zhǐ Shù Wā)
Etymology: The specific epithet is from the small size and the ecological habits of this species, means as the small and cute nymph in the forest, “silvae” as woods or forests, “naias” as nymph.
Diagnosis: Small sized frog of genus Kurixalus; canthus prominent; fore interorbital distance slightly shorter than upper eyelid length, hind interorbital distance approximately equal to upper eyelid length; orbital length longer than tip of snout-eye distance; distinct tympanum; vomerine teeth present with 6 teeth each side; tongue notched behind; fingers almost free with rudimentary webbings, toes almost one-second webbed; tips of all digits dilated but all smaller than tympanum; large subarticular tubercles; wrist much beyond the tip of snout; rounded palmar tubercles and some distinct small palmar tubercles; tibio-tarsal articulation reaching between central orbital and cheek; an small oval inner metatarsal tubercle; no outer tubercle apparent; rough skin above with distinct irregular warts, smooth skin on body sides with several small warts, granular ventral surface; yellowish brown dorsal surface with a large hourglass shaped dark pattern; light yellow body sides, ventral area from head to the upper area of whitish chest with dark marking marbled on lower jaw and a pair of large symmetrical dark blotches on chest, from lower area of chest to dark purple abdomen with confluent warts, some black warts.
2.2 Description of holotype CIB 118049
2.2.1 Habitus
Rather slender and flattened; small-sized frog of genus Kurixalus.
2.2.2 Head
Moderate, depressed; blunt snout, distinctly protruding (ESL 15.01% of SVL), have distinct ridges, projecting beyond mandible, mouth extending to the middle area of vertical line of tympanum; prominent can thus, oblique and slightly concave loreal region; HL 40.78% of SVL, longer than wide (HL 35.98% of SVL; HW 88.24% of HL; HD 48.25% of HL); slightly nicked inferior aspect of snout, distinct inner margin of tip of mandible notch; large eye (OL43.25% of HL); pointed nostril, swelled, oval, cut in lateral direction on the snout, nostrils nearer to tip of the snout than to eyes (END 51.75% of ESL), narrow and concave internarial area (IND 50.70% of OL); eye to nostril distance less than eye diameter (END 44.04% of OL); circular pupil; upper eyelid slightly longer than the anterior interorbital and less than posterior interorbital (FIOD 91.94% of UEL; UEL 94.48% of HIOD; HIOD 57.61% of HW); tympanum’s length distinctly shorter than eye and slightly larger than the wide of third finger disk (TDH 44.21% of OL; FD3 88.89% of TDH), separated from eye by distance (TED) 56.35% of tympanum length(TDH); distinct supratympanic fold, from the posterior orbital along the upper edge of tympanum backwards to the upper area of shoulder; distinct vomerine teeth, with two groups on each inner side of choana, 6 teeth formed a group; large tongue (almost occupied half of mouth), pyriform, smooth dorsal surface, distinctly bifid and free posteriorly (Figure 4).
 Figure 4 Dorsal, lateral, ventral and mouth view of the head of specimen CIB 118049 Note: Photographs by Mian HOU |
2.2.3 Body
Flattened and slender, have a concave area along with vertebral line from occipitalis to waist, uniform small ridges and warts on dorsal area; distance from gular to the lower edge of the vent is 59.22% that between the latter and tip of snout; distance from axilla to groin is 52.29% of SVL; distance between axilla to groin is 88.30% of the length from gular to the lower edge of the vent (Figure 5).
 Figure 5 Dorsal, lateral, ventral and axilla-groin view of the specimen CIB 118049 Note: Photographs by Mian HOU |
2.2.4 Forelimbs
Slim and long, 62.16% of SVL, much shorter than hindlimbs (FLL 47.18% of HLL), the wrist much exceed the tip of snout and the elbow reaching the nostril when the forelimbs adpressed forward; relative length of fingers: I<IV<Ⅱ<III; finger tips dilated into large, rounded and flattened disks, with grooves separating dorsum of disks from venter; larger digital disk on finger III (F3L 35.64% of FLL), smallest on first finger (F1L 24.24% of FLL); one enlarged oval metacarpal tubercle on the base of inner first finger, the other two rather large metacarpal tubercles on the base of outer two fingers, large and distinct subarticular tubercles, have some small indistinct supernumerary tubercles on central area of palm between the base of outer three fingers and metacarpal tubercles, especially the third finger; formula of subarticular tubercles: 1, 1, 2, 1; outer palmar tubercles strongly developed, oval and long, and without inner palmar tubercle; outer side of fourth finger fringed, its outer edge distinctly serrated; webbing between fingers slightly developed, about 30% between base up to proximal subarticular tubercles (Figure 6).
 Figure 6 Forelimbs of specimen CIB 118049: dorsal and ventral view of the right hand Note: Photographs by Mian HOU |
2.2.5 Hindlimbs
Strong and long, exceed 2 times length of forelimb and 1.32 times longer than body length (FLL 47.18% of HLL; SVL 75.69% of HLL); TBL 40.41% of SVL; FOT 41.09% of SVL; FL 36.45% of SVL; tibio-tarsal articulation reaching cheek when hindlimbs adpressed forward; toes webbed, relative length I<Ⅱ<V<III<IV (T1L 9.07% of SVL and 6.88% of HLL; T4L 24.01% of SVL and 18.22% of HLL); toes with rounded disks, tips of toes dilated into flattened disks with grooves that separate dorsum of disks from ventrum, smaller than the ones on fingers; large subarticular tubercles roundish: 1, 1, 2, 3, 2; inner metatarsal tubercle ovaled and raised (IMTL 64.51% of T1L); without outer metatarsal tubercle; have small warts on the inner aspect of metatarsal area; outer side of fifth toe fringed, its outer edge distinctly serrated; webs between toes well developed: I(1)-(1)II(0)-(2)III(1)-(2)IV(2)-(0)V (Figure 7).
 Figure 7 Hindlimbs of specimen CIB 118049 Note: Dorsal (A) and ventral (C) view of the right foot; cloacal area (B); posterior (D) and ventral view (E) of the thighs. Photographs by Mian HOU |
2.2.6 Skin
Above rough with slightly irregular gland ridges and warts studded with small granular, with some slightly enlarged warts on top of each supraorbital; some distinctly enlarged glands on the area from the posterior orbital along the upper edge of tympanum backwards to the upper area of shoulder, formed as supratympanic fold; the warts on dorsal area are all uniform, except one distinct enlarged on middle point of the vertical line between the anterior edge of orbits, it’s a pineal gland; lateral parts smooth and scattered some weakly warts; distinct irregularly big warts and ridged glands on the limbs; granular ventral surface, more so on venter; anus area have enlarged thickened flat granules (Figure 4; Figure 5; Figure 6; Figure 7).
2.2.7 Color
The ground coloration of dorsum is dark brown or coffee; some clusters of light brown or orange enlarged gland ridges on the lateral part from the posterior edge of tympanum over the shoulder backwards to the upper edge of cloacae; dark brown enlarged gland ridges on back; the unconspicuous band on forelimbs, the all dark brown gland ridges and conical tubercles on band, other light brown or orange conical tubercles; the unconspicuous bands on hindlimbs, have four weakly developed bands on the inner part of thigh and outer side of tibia, the brown enlarged gland ridges, other dark glands; the dark bluish part between axilla and groin, few grayish white warts on the upper part, clouded grayish white patterns in the lower part contact with ventral patterns; dark blue cloacae area, with some irregular orange conical glands around there; more grayish white and dark blue conical diffused glands on opisthenars, one or two grayish white bands on first and second fingers, disks grayish white above; grayish white ground color of insteps and webs, every section of toes all have dark blue broad band, these bands linked interrupted through the web, some orange glands in outer side of fourth and fifth toes, grayish white disks above; dark blue ventral surface with numerous irregular grayish white patterns and speckles on it; black pupil, pale bluish white iris with irregular black patterns (Figure 4; Figure 5; Figure 6; Figure 7).
2.2.8 Sexual dimorphism
Male distinctly smaller than female which SVL about 50% of latter; single internal vocal sac in male and absent in female; male without lineal masculine; female with prominent tip of snout as opposed of slightly prominent in male (Figure 8).
 Figure 8 Sexual dimorphisms of Kurixalus silvaenaias Note: A. the vocal sac of male; B. nuptial color of male in breeding period; C. dorsal view of male; D. pregnant female; E. a pair of K. silvaenaias in hugs; F. dorsal view of female. Photographs by Miao Jing-ling (A, D, B, E) and Mian HOU(C, F) |
2.2.9 Variation
All type specimens are very similar in appearance only with slightly variation of color depth among them.
2.2.10 Distribution
Currently only known from its type locality (Figure 9).
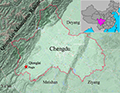 Figure 9 Geographic range of Kurixalus silvaenaias Note: The locations of K. silvaenaias: red pentacle is the type locality Qionglai |
2.2.11 Natural history and ecology
This species inhabits the bamboo broad-leaved mixed forest in a small hilly with altitude from 623-725 m, we found 4 aggregation puddles along with a narrow river valley, they are singing in April to May, and we observed their mating in the end of April, almost time there have about 10-20 adult males calling around a puddle, and 1 or 2 females were observed there. Each female laid about 150-200 eggs on land where the bank of the puddle under the bush, and they are washed in puddle by rain later. In captivity, they are fed by small crickets and cockroaches actively, but in favor of latter (Figure 10).
 Figure 10 The habitat of Kurixalus silvaenaias on Pingle Town, Qionglai County Note: A, The holistic habitat; B, C, the microhabitat; D and F, a male on leaf and a pregnant female on branch; G and H, a couple of K. silvaenaias in hugs and the laying pair under the bush; F and I, a group of new laid eggs and the first developmental stage of the eggs. Photographs by Jing-ling Miao (A-H) And Mian HOU (I) |
2.2.12 Conservation
Considering the habitat’s situation of this species, we suggest its IUCN status as “EN (Endangered)”, because this tree frog inhabited within a small area with few populations, and the known populations are all in lower elevation hills where facing a lot of fast habitat reduction by human activities constantly, such as agricultural development and tourism development etc.
2.2.13 Comparisons
Morphologically the new species can be distinguished from its sister species, K. idiootocus, by larger body size in males (29.6-32.9 mm vs. 24.9-29.3 mm), single internal vocal sac (vs. single external vocal sac), light yellow nuptial pad on the base of first finger in males (vs. white nuptial pads on the first and second fingers in males), and throat coarsely granulated (vs. finely granulated).
Kurixalus silvaenaias sp. nov. is distinguishable from K. raoi by dorsal surface yellowish brown with a large hourglass shaped dark brown marking with wide black edge (vs. dorsal surface grayish-brown with two dark brown arcuate stripes), snout obtuse and rounded with no dermal prominence on tip (vs. obviously pointed), and throat coarsely granulated (vs. finely granulated).
Kurixalus silvaenaias sp. nov. obviously differs from other eighteen congeners including K. absconditus, K. appendiculatus, K. baliogaster, K. banaensis, K. berylliniris, K. bisacculus, K. chaseni, K. eiffingeri, K. gracilloides, K. hainanus, K. lenquanensis, K. motokawai, K. naso, K. odontotarsus, K. verrucosus, K. viridescens, K. wangi, and K. yangi by the presence of pair of large symmetrical dark blotches on chest (vs. absent).
The new species can be further distinguished from K. lenquanensis by larger body size in males (29.6-32.9 mm vs. 25.0-28.9 mm); from K. absconditus by presence of outer metacarpal tubercles (vs. absent), absence of green speckles on dorsal surface (vs. present), coarsely granular throat (vs. smooth), weak mandibular symphysis (vs. prominent), and chin completely clouded with black or scattered marbled with large black speckles (vs. lower jaw nearly immaculate as opposed to only scattered a few small dark speckles); from K. appendiculatus by absence of green speckles on dorsum (vs. present); from K. baliogaster by tuberculated dorsal and lateral skin (vs. smooth), presence of tubercles on eyelids (vs. absent), granular throat (vs. smooth), and presence of crenulated dermal fringes on limbs (vs. absent); from K. banaensis by presence of vomerine teeth (vs. absent), absence of green speckles on dorsum (vs. present), and tuberculate flanks (vs. smooth); from K. berylliniris by pale white iris with irregular black lines, golden speckles and patterns (vs. emerald to light green) and nuptial pad less developed (vs. nuptial pad greatly expanded); from K. bisacculus by single internal vocal sac (vs. two external vocal sacs); from K. chaseni by absence of green speckles on dorsum (vs. present), tibio-tarsal articulation reaching eye or cheek (vs. reached the tip of snout), and chin completely clouded with black or scattered marbled with large black speckles (vs. lower jaw nearly immaculate); from K. eiffingeri and K. wangi by nuptial pad less developed (vs. nuptial pad greatly expanded); from K. gracilloides by dark purple abdomen (vs. off-white); from K. hainanus by larger body size (29.6-32.9 mm vs. 23.2-28.4 mm), absence of green speckles on dorsum (vs. present), and dark purple abdomen (vs. white scattered with large dark blotches); from K. motokawai by smaller body size (29.6-32.9 mm vs. 30.1-39.1 mm), presence of vomerine teeth (vs. absent), and absence of green speckles on dorsum surface (vs. present); from K. naso by absence of green speckles on dorsum surface (vs. present) and dark purple abdomen (vs. white scattered with dark spots); from K. odontotarsus by smaller body size in males (29.6-32.9 mm vs. 32.1-34.3 mm), absence of green speckles on dorsum surface (vs. present), and dark purple abdomen (vs. white scattered with dark spots); from K. verrucosus by coarsely granular throat and chest (vs. smooth), absence of green speckles on dorsal surface (vs. present), and dark purple abdomen (vs. white scattered with dark spots); from K. viridescens by yellowish-brown dorsal surface (vs. uniformly green); from K. yangi by smaller body size (29.6-32.9 mm vs. 31.6-34.7 mm in males), absence of green speckles on dorsum surface (vs. present), and dark purple abdomen (vs. white scattered with small brown spots).
3 Discussion
The frilled swamp treefrog of genus Kurixalus are widely distributed in area of South and West China as far north as Medog of Tibet, but has never been reported from Sichuan, China. The new species of Kurixalus described here is the first record of Kurixalus in Sichuan Province, which improves our understanding of amphibian diversity of Sichuan Province on both species and genus levels.
The new species is the fourth Asian mainland Kurixalus species phylogenetically clustered together with its congeneric members on the East Asian island clade, the other three species include K. lenquanensis from Yunnan, K. raoi from Guizhou, and K. gracilloides from Vietnam. Yu et al. (2020) revealed that the genus Kurixalus has immigrated into the Taiwan Island from Asian mainland via two long-distance colonization events during the late Miocene and Pleistocene. We speculate that the common ancestor of the new species, K. lenquanensis, K. raoi, K. gracilloides, and the four species on Taiwan and Ryukyu (K. idiootocus, K. berylliniris, K. eiffingeri, and K. wangi) might have originated in southwestern China and underwent radiations northward to Sichuan and southeastward to Taiwan via long-distance dispersal. More analyses are needed to unveil the biogeographic origin of Kurixalus because Nguyen et al. (2020) inferred that the ancestor of species from Taiwan Island likely inhabited both Taiwan and Asian mainland (Figure 11).
 Figure 11 Comparison of the species within Kurixalus sensu stricto of topotypes Note: A, K. eiffingeri; B, K. berylliniris; C, K. wangi; D, K. idiootocus; E, K. gracilloides; F, K. silvaenaias; G, K. lenquanensis and H, K. raoi. Photographs by Chung-wei YOU (A, B, D), Guo-qing JI (C), Tan Van NGUYEN (E), Jing-ling Miao (F), Guo-hua YU (G, H) |
The taxonomy of Kurixalus needs further investigation (Yu et al., 2017b; Zeng et al., 2021). Taking K. silvaenaias sp. nov. into account, now a total of 21 species are placed in the genus Kurixalus and 12 of them are known from China. The new record of Kurixalus in Sichuan, China represents the northernmost distribution of Kurixalus so far and greatly expands the distributional range of Kurixalus. Considering the huge geographical discontinuity in distribution between K. silvaenaias sp. nov. and its congeners (Figure 1), we suspect that potentially more Kurixalus species are waiting to be found in the region of distributional gap. Similarly, probably more Kurixalus species allied to the East Asian islands clade will be found from South China as expected by Yu et al. (2017a) and Zeng et al. (2021).
Authors’ contributions
HM drafted the manuscript and carried out the molecular genetic studies, participated in the sequence alignment. PXP participated in the sequence alignment. MJL participated in the design of the study and performed the statistical analysis. LS conceived of the study, and participated in its design and coordination and helped to draft the manuscript. LPP participated in reviewed and revised the manuscript. ONL participated in the review and revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (32060114), Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education (ERESEP2020Z22), Guangxi Key Laboratory of Rare and Endangered Animal Ecology, Guangxi Normal University (19-A-01-06) to Guo-hua YU, the Hainan Tropical Wildlife Park and Botanical Garden, and the Project of Nanhai Series of Talent Cultivation Program. We are grateful to Researchers Liang FEI and Chang-yuan YE for help to identify the new species and study it’s tadpoles, Patrick DAVID and Gernot VOGEL for corrected the Latin word, Chung-wei YOU and Tan Van NGUYEN for providing the important photos of Kurixalus species from Taiwan, China and K. gracilloides from Vietnam, Ji-wei WANG helped for field survey.
Annandale N., 1912, Zoological results of the Abor expedition, I. Batrachia, Rec. Indian Mus., 8, 7-36
https://doi.org/10.5962/bhl.part.1186
Boettger O., 1895, Neue Frösche und Schlangen von den Liukiu-Inseln, Zoologischer Anzeiger, 18, 266-270
Boulenger G. A., 1893, Concluding report on the reptiles and batrachians obtained in Burma by Signor L. Fea dealing with the collection made in Pegu and the Karin Hills in 1887-88, Annali del Museo Civico di Storia Naturale di Genova, 13, 304-347
Bourret R., 1939, Notes herpétologiques sur I’Indochine française, XVII, reptiles et batraciens reçus au Laboratoire des Sciences Naturelles de I’Université au cors de I’année 1938, descriptions de trois espèces nouvelles, Ann. Bull. Gén. I’Inst. Publ., 6, 13-34
Du L. N., Liu S., Hou M., and Yu G. H., 2020, First record of Theloderma pyaukkya Dever, 2017 (Anura: Rhacophoridae) in China, with range extension of Theloderma moloch (Annandale, 1912) to Yunnan, Zool. Res., 41(5), 576-580
https://doi.org/10.24272/j.issn.2095-8137.2020.083
PMid:32692491 PMCid:PMC7475012
Frost D. R., 2021, Amphibian species of the world 6.1, an online reference, New York: American Museum of Natural History. available from URL: https://amphibiansoftheworld. amnh. org/index. php
Günther A. C. L. G., 1858, Neue batrachier in der sammlung des britischen museums, Archiv für Naturgeschichte, 24, 319-328
https://doi.org/10.5962/bhl.part.5288
Huelsenbeck J. P. and Ronquist F., 2001, MrBayes. Bayesian inference of phylogeny, Bioinformatics, 17, 754-755
https://doi.org/10.1093/bioinformatics/17.8.754
PMid:11524383
Inger R. F., Orlov N. L., and Darevsky I. S., 1999, Frogs of Vietnam: a report on new collections, Fieldiana Zool., 92: 1-46
Kuramoto M. and Wang C. S., 1987, A new rhacophorid treefrog from Taiwan, with comparisons to Chirixalus eiffingeri (Anura, Rhacophoridae), Copeia, 1987(4), 931-942
https://doi.org/10.2307/1445556
Mediyansyah M., Hamidy A., Munir M., and Matsui M., 2019, A new tree frog of the genus Kurixalus Ye, Fei and Dubois, 1999 (Amphibia: Rhacophoridae) from West Kalimantan, Indonesia, Treubia, 46, 51-72
https://doi.org/10.14203/treubia.v46i0.3790
Nguyen T. T., Matsui M., and Duc H. M., 2014a, A new tree frog of the genus Kurixalus (Anura: Rhacophoridae) from Vietnam, Curr. Herpetol., 33(2), 101-111
https://doi.org/10.5358/hsj.33.101
Nguyen T. T., Matsui M., and Eto K., 2014b, A new cryptic tree frog species allied to Kurixalus banaensis (Anura: Rhacophoridae) from Vietnam, Russ. J. Herpetol., 21(4), 295-302
Nguyen T, V., Duong T. V., Luu K. T., and Poyarkov N. A., 2020, A new species of Kurixalus (Anura: Rhacophoridae) from northern Vietnam with comments on the biogeography of the genus, J. Nat. Hist., 54(1-4), 195-223
https://doi.org/10.1080/00222933.2020.1728411
Posada D. and Crandall K. A., 1998, Modeltest, testing the model of DNAsubstitution, Bioinformatics, 14, 817-818
https://doi.org/10.1093/bioinformatics/14.9.817
PMid:9918953
Smith M. A., 1924, New tree-frogs from Indo-China and the Malay peninsula, Proc. Zool. Soc. Lond., 94(1), 225-234
https://doi.org/10.1111/j.1096-3642.1924.tb01499.x
Taylor E. H., 1962, The amphibian fauna of Thailand, Univ. Kansas Sci. Bull., 43, 265-599
https://doi.org/10.5962/bhl.part.13347
Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin J., and Higgins D. G., 1997, The CLUSTAL X windows interface, flexible strategies for multiple sequence alignment aided by quality analysis tools, Nucl. Acids Res., 25, 4876-4882
https://doi.org/10.1093/nar/25.24.4876
PMid:9396791 PMCid:PMC147148
Wu S. P., Huang C. C., Tsai C. L., Lin T. E., Jhang J. J., and Wu S. H., 2016, Systematic revision of the Taiwanese genus Kurixalus members with a description of two new endemic species (Anura, Rhacophoridae), ZooKeys, 557(3), 121-153
https://doi.org/10.3897/zookeys.557.6131
PMid:26877703 PMCid:PMC4740841
Ye C. Y., Fei L., and Hu S. Q., 1993, Rare and Economic Amphibians of China, Sichuan Publishing House of Science and Technology
Yu G. H., Du L. N., Wang J. S., Rao D. Q., Wu Z. J., and Yang J. X., 2020, From mainland to islands: colonization history in the tree frog Kurixalus (Anura: Rhacophoridae), Curr. Zool., 66(6), 667-675
https://doi.org/10.1093/cz/zoaa023
Yu G. H., Hui H., Rao D. Q., and Yang J. X., 2018, A new species of Kurixalus from western Yunnan, China (Anura, Rhacophoridae), ZooKeys, 770, 211-226
https://doi.org/10.3897/zookeys.770.23526
PMid:30002595 PMCid:PMC6041353
Yu G. H., Rao D. Q., Matsui M., and Yang J. X., 2017b, Coalescent-based delimitation outperforms distance-based methods for delineating less divergent species: the case of Kurixalus odontotarsus species group, Sci. Rep., 7, 16124
https://doi.org/10.1038/s41598-017-16309-1
PMid:29170403 PMCid:PMC5700917
Yu G. H., Wang J. S., Hou M., Rao D. Q., and Yang J. X., 2017a, A new species of the genus Kurixalus from Yunnan, China (Anura, Rhacophoridae), ZooKeys, 694, 71-93
https://doi.org/10.3897/zookeys.694.12785
PMid:29134000 PMCid:PMC5672778
Zeng J., Wang J. S., Yu G. H., and Du L. N., 2021, A new species of Kurixalus (Anura, Rhacophoridae) from Guizhou, China, Zool. Res., 42(2), 227-233
Zhao E. M., Wang L. J., Shi H. T., Wu G. F., and Zhao H., 2005, Chinese rhacophorid frogs and description of a new species of Rhacophorus, Sichuan J. Zool., 24(3), 297-300
. PDF(4414KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Mian Hou
. Xiaopeng Peng
. Jingling Miao
. Shuo Liu
. Pipeng Li
. Nikolai L. Orlov
Related articles
. Kurixalus silvaenaias sp. Nov.
. Chengdu
. China
. New species
Tools
. Email to a friend
. Post a comment


